-
Name
5-CHLORO-N-METHYL-2-NITROBENZENAMINE
- EINECS
- CAS No. 35966-84-8
- Article Data36
- CAS DataBase
- Density 1.406 g/cm3
- Solubility
- Melting Point 104-107 °C
- Formula C7H7ClN2O2
- Boiling Point 318.1 °C at 760 mmHg
- Molecular Weight 186.598
- Flash Point 146.2 °C
- Transport Information
- Appearance yellow to orange-yellow powder
- Safety 38-28
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Aniline,5-chloro-N-methyl-2-nitro- (6CI);4-Chloro-2-(methylamino)nitrobenzene;5-Chloro-N-methyl-2-nitroaniline;N-Methyl-5-chloro-2-nitroaniline;NSC 86687;
- PSA 57.85000
- LogP 2.88610
Synthetic route

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 40℃; for 22h; Product distribution / selectivity; | 100% |
| With triethylamine In tetrahydrofuran at 40℃; for 22h; Inert atmosphere; | 100% |
| In water; dimethyl sulfoxide at 27 - 37℃; for 6h; | 90% |
-
-
1635-61-6
5-chloro-2-nitroaniline

-
-
74-88-4
methyl iodide

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; | 100% |
| Stage #1: 5-chloro-2-nitroaniline With sodium hydride In tetrahydrofuran; paraffin oil at 0℃; for 0.25h; Stage #2: methyl iodide In tetrahydrofuran; paraffin oil at 20℃; for 2h; | 95% |
-
-
1635-61-6
5-chloro-2-nitroaniline

-
-
77-78-1
dimethyl sulfate

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In water; toluene at 20℃; Methylation; | 96% |
| Stage #1: 5-chloro-2-nitroaniline With potassium tert-butylate In DMF (N,N-dimethyl-formamide) at 0℃; for 1h; Stage #2: dimethyl sulfate In DMF (N,N-dimethyl-formamide) at 25℃; for 1.5h; |
-
-
700-37-8
4-chloro-2-fluoro-nitrobenzene

-
-
74-89-5
methylamine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| In ethanol at 0℃; for 2.25h; | 92.7% |
| In ethanol; water for 1h; | 33% |
| In tetrahydrofuran; dimethyl sulfoxide at 20℃; for 24h; Product distribution / selectivity; | |
| In tetrahydrofuran at 20℃; | |
| In tetrahydrofuran at 20℃; for 1h; Microwave irradiation; Sealed tube; |
-
-
77-78-1
dimethyl sulfate

-
-
174264-60-9
5-chloro-2-nitro-N-trifluoroacetylaniline

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In toluene Ambient temperature; | 90% |

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 88% |
-
-
610-40-2
3,4-dinitro-chlorobenzene

-
-
74-89-5
methylamine

-
A
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
B
-
61149-80-2
N-methyl-3,4-dinitroaniline

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; | A 60% B n/a |


-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
610-40-2
3,4-dinitro-chlorobenzene

-
-
74-89-5
methylamine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With ethanol at 25℃; | |
| With sodium hydroxide; ethanol | |
| With sodium hydroxide; ethanol |
-
-
67-56-1
methanol

-
-
72693-63-1
3'-Chlor-5'-nitro-N-methyltoluanilid

-
A
-
99-75-2
4-methyl-benzoic acid methyl ester

-
B
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With sodium at 99.9℃; Rate constant; |

-
-
1635-61-6
5-chloro-2-nitroaniline

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sat. NaHCO3 / CH2Cl2 / Ambient temperature 2: 90 percent / benzyltriethylammonium chloride, aq. NaOH / toluene / Ambient temperature View Scheme | |
| With NaH In N,N-dimethyl-formamide |
-
-
55775-97-8
2,6-dichloro-3-nitrobenzoic acid

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 97 percent / ethanol; H2O / 2 h / Heating 2: 88 percent / 18percent aq. HCl View Scheme |
-
-
7006-52-2
3-chloro-N-methylaniline

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: alcohol / Einleiten von nitrosen Gasen 2: fuming hydrochloric acid View Scheme |
-
-
108-42-9
3-chloro-aniline

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: beim Methylieren 2: alcohol / Einleiten von nitrosen Gasen 3: fuming hydrochloric acid View Scheme |

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; dichloromethane |
-
-
855876-28-7
acetic acid-(5-chloro-N-methyl-2-nitro-anilide)

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With sulfuric acid Heating; |
-
-
74-89-5
methylamine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran |
-
-
107-03-9
1-thiopropane

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
80983-49-9
N-methyl-2-nitro-5-(propylthio)aniline

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; ethylene glycol at 115℃; for 4h; | 96.8% |
-
-
141-32-2
acrylic acid n-butyl ester

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
1370023-74-7
n-butyl 3-methylamino-4-nitrocinnamate

| Conditions | Yield |
|---|---|
| With palladium diacetate; N-ethyl-N,N-diisopropylamine; lithium chloride at 110℃; for 12h; Heck Reaction; Inert atmosphere; | 96% |
| With palladium diacetate; N-ethyl-N,N-diisopropylamine; lithium chloride In N,N-dimethyl acetamide at 110℃; for 12h; Heck Reaction; Inert atmosphere; | 71.8 g |
-
-
141-32-2
acrylic acid n-butyl ester

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
863886-03-7
n-butyl (E)-3-[4-nitro-3-(methylamino)phenyl]prop-2-enoate

| Conditions | Yield |
|---|---|
| With palladium diacetate; N-ethyl-N,N-diisopropylamine; lithium chloride at 108 - 112℃; for 6h; Heck Reaction; Inert atmosphere; | 95% |
| With N-Methyldicyclohexylamine; tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine In 1,4-dioxane at 110℃; | 81% |
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; N-Methyldicyclohexylamine In 1,4-dioxane at 110℃; for 72h; Inert atmosphere; Sealed tube; | 81% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
123-30-8
4-amino-phenol

-
-
433225-99-1
4-(3-methylamino-4-nitrophenoxy)aniline

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 90℃; | 94% |
| With NaH In N,N-dimethyl-formamide | 94% |
-
-
3096-70-6
3,5-dimethyl-4-aminophenol

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
881883-40-5
N-[5-(4-amino-3,5-dimethylphenoxy)-2-nitrophenyl]-N-methylamine

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol; potassium tert-butylate In N,N-dimethyl acetamide at 60℃; for 0.5h; Product distribution / selectivity; | 93% |
| With potassium tert-butylate In N,N-dimethyl acetamide at 75 - 80℃; for 1h; Product distribution / selectivity; | 80% |
| In N,N-dimethyl acetamide; water |
-
-
123-75-1
pyrrolidine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| for 0.75h; Heating; | 92% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
69397-93-9
N-methyl-5-methoxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; DMF (N,N-dimethyl-formamide) at 55℃; for 2h; | 90% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
1635-61-6
5-chloro-2-nitroaniline

-
-
69397-93-9
N-methyl-5-methoxy-2-nitroaniline

| Conditions | Yield |
|---|---|
| With sodium methylate; dimethyl sulfate In N-methyl-acetamide; methanol; water | 90% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
84859-27-8
5-chloro-N1-methylbenzene-1,2-diamine

| Conditions | Yield |
|---|---|
| With tin(II) chloride dihdyrate In ethyl acetate for 16h; Reflux; | 88% |
| With ethanol; nickel Hydrogenation; | |
| With hydrogenchloride; tin(ll) chloride |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
154334-40-4
(S)-(+)-N-<5-Chlor-2-nitro-phenyl>-N-methyl-ethyl-methyl-cyanessigsaeureamid

| Conditions | Yield |
|---|---|
| In pyridine Heating; | 88% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
149-73-5
trimethyl orthoformate

-
-
10406-94-7
1-methyl-6-chloro-1H-benzo[d]imidazole

| Conditions | Yield |
|---|---|
| With hydrogen; pyridinium p-toluenesulfonate; palladium on activated charcoal In ethyl acetate at 20℃; under 2585.74 Torr; for 6h; | 88% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
154334-39-1
(R)-(-)-N-<5-Chlor-2-nitro-phenyl>-N-methyl-ethyl-methyl-cyanessigsaeureamid

| Conditions | Yield |
|---|---|
| In pyridine Heating; | 85% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
140-53-4
p-chlorobenzyl cyanide

-
-
914401-82-4
C15H12ClN3O2

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride | 85% |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In tetrahydrofuran; water at 50℃; for 48h; | 66.8% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 100 - 110℃; for 4.5h; Time; | 83.31% |
-
-
110-89-4
piperidine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| for 1.5h; Heating; | 82% |
-
-
576-24-9
2,3-dichlorophenol

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
1234449-75-2
5-(2,3-dichlorophenoxy)-N-methyl-2-nitroaniline

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; N,N-dimethyl-formamide at 125℃; for 3h; | 81% |
-
-
109-01-3
1-methyl-piperazine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| for 4h; Heating; | 80% |
| at 20 - 90℃; for 18h; |
-
-
5344-97-8
3,5-dimethyl-4-nitrophenol

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl acetamide at 20 - 140℃; for 2.25h; | 80% |
| With hydrogenchloride In N,N-dimethyl acetamide |
-
-
110-91-8
morpholine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

| Conditions | Yield |
|---|---|
| for 7h; Heating; | 74% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
299176-17-3
N-(2-nitro-5-chlorophenyl)-N-methylcarbamic acid t-butyl ester

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran at 20℃; for 16h; | 73.1% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
153899-88-8
N-<5-Chlor-2-nitro-phenyl>-N-methyl-ethyl-methyl-cyanessigsaeureamid

| Conditions | Yield |
|---|---|
| In pyridine Heating; | 71% |
-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
128796-39-4
4-trifluoromethylphenylboronic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate; tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide at 100℃; for 17h; Suzuki coupling; | 68% |
-
-
50-47-5
desipramine

-
-
35966-84-8
4-chloro-2-methylaminonitrobenzene

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
174264-64-3
4-bromo-2-(1H-1,2,4-triazol-1-ylmethyl)phenol

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; water; ethyl acetate | 62.5% |

Benzenamine,5-chloro-N-methyl-2-nitro- Specification
The Benzenamine,5-chloro-N-methyl-2-nitro-, with the CAS registry number 35966-84-8, is also known as 4-Chloro-2-(methylamino)nitrobenzene. This chemical's molecular formula is C7H7ClN2O2 and molecular weight is 186.6. What's more, its systematic name is 5-chloro-N-methyl-2-nitroaniline. It should be sealed and stored in a cool and dry place.
Physical properties of Benzenamine,5-chloro-N-methyl-2-nitro- are: (1)ACD/LogP: 3.37; (2)# of Rule of 5 Violations: 0; (3)#H bond acceptors: 4; (4)#H bond donors: 1; (5)#Freely Rotating Bonds: 2; (6)Polar Surface Area: 49.06 Å2; (7)Index of Refraction: 1.631; (8)Molar Refractivity: 47.3 cm3; (9)Molar Volume: 132.6 cm3; (10)Polarizability: 18.75×10-24cm3; (11)Surface Tension: 52.7 dyne/cm; (12)Density: 1.406 g/cm3; (13)Flash Point: 146.2 °C; (14)Enthalpy of Vaporization: 55.96 kJ/mol; (15)Boiling Point: 318.1 °C at 760 mmHg; (16)Vapour Pressure: 0.000369 mmHg at 25°C.
Preparation of Benzenamine,5-chloro-N-methyl-2-nitro-: this chemical can be prepared by 2-fluoro-4-chloro-nitrobenzene and methylamine. This reaction will need solvents ethanol, H2O with the reaction time of 1 hour. The yield is about 33%.
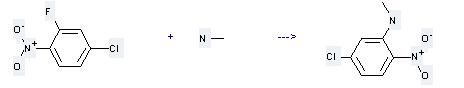
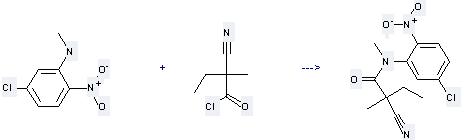
Uses of Benzenamine,5-chloro-N-methyl-2-nitro-: it can be used to produce N-(5-chloro-2-nitro-phenyl)-2-cyano-2,N-dimethyl-butyramide by heating. It will need solvent pyridine. The yield is about 71%.
When you are using this chemical, please be cautious about it as the following:
It is harmful if swallowed. After contact with skin, you must wash immediately with plenty of ... (to be specified by the manufacturer). In case of insufficient ventilation, you should wear suitable respiratory equipment.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CNC1=C(C=CC(=C1)Cl)[N+](=O)[O-]
(2)InChI: InChI=1S/C7H7ClN2O2/c1-9-6-4-5(8)2-3-7(6)10(11)12/h2-4,9H,1H3
(3)InChIKey: YWJPRGWHZDSXML-UHFFFAOYSA-N
Related Products
- Benzenamine, 2-(1,1-dimethylethyl)-6-methyl-
- Benzenamine, 2, 6-dimethyl-3-(methylsulfonyl)-
- Benzenamine, 2,6-diethyl-N-(phenylmethylene)-
- Benzenamine, 2-bromo-3,5-difluoro-
- Benzenamine, 2-bromo-5-chloro-4-methyl-
- Benzenamine, 2-bromo-6-(trifluoromethyl)-
- Benzenamine, 2-bromo-6-chloro-
- Benzenamine, 2-ethenyl-
- Benzenamine, 2-ethoxy-4-nitro-
- Benzenamine, 2-ethyl-N-(2-methoxyethyl)-6-methyl-
- 35967-24-9
- 35967-49-8
- 35969-51-8
- 35969-54-1
- 35970-34-4
- 359-70-6
- 35970-79-7
- 35970-80-0
- 35971-70-1
- 35973-14-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View