-
Name
Benzoyl peroxide
- EINECS 202-327-6
- CAS No. 94-36-0
- Article Data69
- CAS DataBase
- Density 1.254 g/cm3
- Solubility insoluble in water
- Melting Point 105 °C(lit.)
- Formula C14H10O4
- Boiling Point 349.747 °C at 760 mmHg
- Molecular Weight 242.231
- Flash Point 154.239 °C
- Transport Information UN 3108
- Appearance white powder or crystals
- Safety 53-17-26-36/37-45-60-36/37/39-3/7-14A-14-47-35-7-61
- Risk Codes 8-36/37/38-43-36-2-7-1-51/53-21/22-62-50-61
-
Molecular Structure
-
Hazard Symbols
 E,
E, Xi
Xi
- Synonyms Nyper BMT;Nayper B and BO;Nyper BO;Benzoyl superoxide;Desquam-X;Oxy-5;Cadox 40E;Sanperox BPO;Benoxyl (5&10) Lotion;Benzoyl Peroxide,BPO;Crosslink agent BPO PERKADOX L;Di-Benzoyl Peroxide;Lucidol B 50;Clear By Design;Abcat 40;Dibenzoyl peroxide 75% with water;Benzoylperoxide;Benzoyl Peroxide 50%;Diphenylglyoxal peroxide;benzoyl benzenecarboperoxoate;Quinolor compound;Benoxyl;Desquam X;Persa-Gel;Nyper FF;Superox 744;Cadox B;Desquam E;Peroxide,dibenzoyl;PanOxyl;Component of Oxy-5;Component of Vanoxide;TC 1 (peroxide);Lucidol G 20;Nyper B;Lucidol 78;Vanoxide;Benzagel;Benzac W;Peroxide, dibenzoyl;Brevoxyl;143928-58-9;Luperox FL;Oxy 5;Nayper BO;Superox 46-750;Novadelox;Resdan Akne;Peroxyde de benzoyle [French];Cadox B 75W;Aztec BPO;B 75W;Luperco AST;Perossido di benzoile;Benzoyl peroxide [USAN];Debroxide;Nyper FF-K;Dibenzoylperoxid;Oxy-10;Cadat BPO;Benzol peroxide;Cadet BPO 78W;Cadox B-CH 50;Oxylite;Incidol;Acnegel;Lucidol KL 50;Benzoic acid, peroxide;Xerac;Fostex;Benox 50;BPO;Duresthin 5;Chaloxyd BP 50FT;Oxy-10 Cover;Benzashave;Persadox;Benzoylperoxid [German];Benzoylperoxyde [Dutch];Lucidol-70;Nyper BS;Clearasil Antibacterial Acne Lotion;Nyper BMT 40;Benzoylperoxyde;Eloxyl;W 75;Dibenzoyl peroxide;Cadox B 70W;Cadox BS;G20;Diphenylperoxyanhydride;pHisoAc BP;Lucidol 98;Nyper BW;Lucidol 50P;Acne-Aid Cream;Cadox B 50P;G 20;Lucidol (peroxide);Perossido di benzoile [Italian];BZF-60;Benzoperoxide;Dibenzoylperoxid [German];Benzagel 10;Aksil 5;Lucidol;Dry and Clear;Clearasil BP Acne Treatment Cream;Cadox B 40E;Topex;Nyper NS;Theraderm;Epi Clear Antiseptic Lotion;Norox bzp-250;Lucidol BW 50T;Benzamycin;Benzaknen;Desanden;Stri-dex B.P.;Norox bzp-C-35;Fostex BPO;Xerac BP;Lucidol KH 50;Akneroxide L;Lucidol 70;Mytolac;Loroxide;Acetoxyl;Akneroxid 5;Benbel C;Garox;Dibenzoylperoxyde [Dutch];Dibenzoylperoxyde;Benzoylperoxid;Peroxyde de benzoyle;Asidopan;
- PSA 52.60000
- LogP 2.61540
Synthetic route

| Conditions | Yield |
|---|---|
| With Eosin Y; triphenylphosphine In ethanol at 20℃; Irradiation; | 95% |
| With dihydrogen peroxide; dicyclohexyl-carbodiimide In diethyl ether; dichloromethane at 0℃; for 2h; Mechanism; | 90% |
| With dihydrogen peroxide; dicyclohexyl-carbodiimide In diethyl ether; dichloromethane at 0 - 2℃; | 90% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide In 1,1,2-Trichloro-1,2,2-trifluoroethane at 0 - 5℃; | 93% |
| With 1,2-ethanediol, dibenzoate; dihydrogen peroxide; sodium 4-dodecylbenzenesulfonate; sodium hydroxide In 2-Methylpentane; water at 20℃; for 0.5h; Reagent/catalyst; Solvent; Temperature; | 84.7% |
| With dihydrogen peroxide; sodium carbonate |
-
-
93-59-4
Perbenzoic acid

-
-
71-43-2
benzene

-
A
-
93-99-2
benzoic acid phenyl ester

-
B
-
92-52-4
biphenyl

-
C
-
65-85-0
benzoic acid

-
D
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| cobalt(III) acetylacetonate at 24.9℃; for 5h; Mechanism; Product distribution; Thermodynamic data; Ea; other metal 2,4-pentandioates (Co(II), Fe(III), Mn(III), Cr(III)).; | A 6% B 8% C 90% D 2% E n/a |


| Conditions | Yield |
|---|---|
| With oxygen; ozone; lithium chloride In tetrachloromethane at 40℃; | A n/a B n/a C 83.2% |


| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; for 6h; | A 27% B 22% C n/a |

-
-
26274-19-1
5-bromo-3,4-dihydro-2H-pyran

-
A
-
32513-73-8
3-bromo-2-ethoxy-5,6-dihydro-2H-pyran

-
B
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With tetrachloromethane; N-Bromosuccinimide Erwaermen des Reaktionsprodukts mit Natriumaethylat in Aethanol und Aether; |

| Conditions | Yield |
|---|---|
| With sodium carbonate at 50℃; |
-
-
79-21-0
peracetic acid

-
-
127-09-3
sodium acetate

-
-
98-88-4
benzoyl chloride

-
A
-
644-31-5
acetyl benzoyl peroxide

-
B
-
94-36-0
dibenzoyl peroxide


| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate |

| Conditions | Yield |
|---|---|
| With barium peroxide | |
| With potassium hydroxide; dihydrogen peroxide | |
| With dioxygenbis(triphenylphosphine)platinum(0) In dichloromethane for 2h; | 9 % Chromat. |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; dihydrogen peroxide at 0℃; |

| Conditions | Yield |
|---|---|
| at 0℃; |
-
-
93-59-4
Perbenzoic acid

-
-
1076-43-3
benzene-d6

-
A
-
20637-23-4
2,3,4,5,6-pentadeuterio-biphenyl

-
C
-
65-85-0
benzoic acid

-
D
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| cobalt(III) acetylacetonate at 25℃; Mechanism; Product distribution; |

-
-
52947-05-4
[α-13C]benzoyl chloride

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With sodium peroxide |
-
-
56-23-5
tetrachloromethane

-
-
100-52-7
benzaldehyde

-
A
-
65-85-0
benzoic acid

-
B
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| at 0℃; |


-
-
7722-84-1
dihydrogen peroxide

-
-
93-97-0
benzoic acid anhydride

-
A
-
93-59-4
Perbenzoic acid

-
B
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| at 0℃; |

-
-
127-09-3
sodium acetate

-
-
98-88-4
benzoyl chloride

-
A
-
644-31-5
acetyl benzoyl peroxide

-
B
-
94-36-0
dibenzoyl peroxide


| Conditions | Yield |
|---|---|
| at 20℃; |

| Conditions | Yield |
|---|---|
| at 25℃; |


| Conditions | Yield |
|---|---|
| at 50℃; |

| Conditions | Yield |
|---|---|
| With water; sodium acetate |


| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate In diethyl ether for 15h; Heating; | 100% |
| With Carbonate buffer In dichloromethane; water at 20℃; pH=10.5; | 94% |
| With dipotassium hydrogenphosphate In N,N-dimethyl-formamide at 20℃; for 12h; Inert atmosphere; | 59% |
-
-
128-08-5
N-Bromosuccinimide

-
-
122670-62-6
2-Bromo-5-(trifluoromethyl)-6-methoxy-1-naphthalene-carboxylic acid

-
-
122670-68-2
2-chloro-6-methoxy-1-methyl-5-(trifluoromethyl)naphthalene

-
-
94-36-0
dibenzoyl peroxide

-
-
122670-63-7
1-bromomethyl-2-chloro-6-methoxy-5-trifluoromethylnaphthalene

| Conditions | Yield |
|---|---|
| In tetrachloromethane | 100% |

-
-
128-08-5
N-Bromosuccinimide

-
-
122670-68-2
2-chloro-6-methoxy-1-methyl-5-(trifluoromethyl)naphthalene

-
-
94-36-0
dibenzoyl peroxide

-
-
122670-63-7
1-bromomethyl-2-chloro-6-methoxy-5-trifluoromethylnaphthalene

| Conditions | Yield |
|---|---|
| In tetrachloromethane | 100% |

-
-
166180-60-5
ethyl [4-methyl-5-(2-thienyl)isoxazol-3-yl]carboxylate

-
-
94-36-0
dibenzoyl peroxide

-
-
166180-61-6
ethyl [4-bromomethyl-5-(2-thienyl)isoxazol-3-yl]carboxylate

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In CCl4was | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: copper hydroxide; dibenzoyl peroxide In water at 60℃; for 0.5h; Inert atmosphere; Stage #2: In water for 5h; Sonication; Inert atmosphere; | 100% |

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With (2R,5R,1'S)-1-(9-anthracenyl)methyl-5-ethylene-2-[1-hydroxy-1-(quinol-4-yl)]methyl-1-azoniabicyclo[2.2.2]octane chloride; potassium hydroxide In water; toluene at -40℃; for 48h; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With (2R,5R,1'S)-1-(9-anthracenyl)methyl-5-ethylene-2-[1-hydroxy-1-(quinol-4-yl)]methyl-1-azoniabicyclo[2.2.2]octane chloride; potassium hydroxide In water; toluene at -40℃; for 48h; Catalytic behavior; Reagent/catalyst; Temperature; enantioselective reaction; | 99% |

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With (2R,5R,1'S)-1-(9-anthracenyl)methyl-5-ethylene-2-[1-hydroxy-1-(quinol-4-yl)]methyl-1-azoniabicyclo[2.2.2]octane chloride; potassium hydroxide In water; toluene at -40℃; for 48h; enantioselective reaction; | 99% |

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With (2R,5R,1'S)-1-(9-anthracenyl)methyl-5-ethylene-2-[1-hydroxy-1-(quinol-4-yl)]methyl-1-azoniabicyclo[2.2.2]octane chloride; potassium hydroxide In water; toluene at -40℃; for 48h; enantioselective reaction; | 99% |

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With (2R,5R,1'S)-1-(9-anthracenyl)methyl-5-ethylene-2-[1-hydroxy-1-(quinol-4-yl)]methyl-1-azoniabicyclo[2.2.2]octane chloride; potassium hydroxide In water; toluene at -40℃; for 48h; enantioselective reaction; | 99% |

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With (+)-N-benzylcinchonineammonium bromide; potassium carbonate In toluene at 25℃; for 24h; Temperature; Reagent/catalyst; | 99% |
| With C35H35N2O3(1+)*Br(1-); potassium carbonate In toluene at 15℃; for 12h; Time; enantioselective reaction; | 98% |
-
-
1403881-80-0
1-indanone-2-carboxylic acid-(3-ethyl)-3-pentyl ester

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With C35H35N2O3(1+)*Br(1-); potassium carbonate In toluene at 15℃; for 12h; enantioselective reaction; | A n/a B 99% |
| With C35H35N2O3(1+)*Br(1-); potassium carbonate In toluene at 15℃; for 24h; Overall yield = 99 %; | A n/a B n/a |


-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at -10℃; for 1h; Inert atmosphere; | 99% |

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at -10℃; for 1h; Inert atmosphere; | 99% |
-
-
74559-43-6
tris[tris(trimethylsilylmethyl)stannyl]praseodymium*1,2-dimethoxyethane

-
-
94-36-0
dibenzoyl peroxide

-
B
-
42202-20-0
((CH3)3SiCH2)3Sn(OCOC6H5)

| Conditions | Yield |
|---|---|
| In benzene soln. of Pr-Sn complex was shaken with soln. of benzoylperoxide at 50°C for 12 h; Sn-Pr complex pptd.; elem. anal.; org. layer was sepd. by distn.; | A 98.4% B 75% |


| Conditions | Yield |
|---|---|
| Stage #1: 6-methylnicotinonitrile; dibenzoyl peroxide With N-Bromosuccinimide In tetrachloromethane for 18h; Heating / reflux; Stage #2: With 4-cyanobenzyl bromide | 98% |
-
-
82477-25-6
bis[tris(trifluoromethyl)germyl]mercury

-
-
94-36-0
dibenzoyl peroxide

-
A
-
82484-05-7
(benzoyloxy)tris(trifluoromethyl)germane

| Conditions | Yield |
|---|---|
| In benzene dry oxygen-free solvents, evacuated glass ampoules; keeping Ge-compd. in benzene and benzoyl peroxide at 50°C (50 h); sepn. of metallic Hg from soln., removal of benzene in vac., distn. (0.01 mm, bath temp. 40°C); elem. anal.; | A 57% B 98% |

-
-
1352166-68-7
tris(5-bromo-2-methoxyphenyl)antimony(III) benzene monosolvate

-
-
94-36-0
dibenzoyl peroxide

-
-
1352166-71-2
((2-methoxy-5-bromophenyl)3Sb(PhC(O)O)2

| Conditions | Yield |
|---|---|
| In benzene mixt. of tris(2-methoxy-5-bromophenyl)antimony benzene monosolvate and benzoyl peroxide in C6H6 was heated for 3 h at 40°C; cooled; solvent removed; elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| In benzene under Ar, benzene soln. of ethyl(bis(pentafluorophenyl)germyl)thallium(I) added to soln. of benzoyl peroxide in benzene, kept for 10 min at 20°C; org. layer decanted, benzene removed in vac., residue recrystd. from hexane, elem. anal.; | A 86% B 97% |


-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With C35H35N2O3(1+)*Br(1-); potassium carbonate In toluene at 15℃; for 12h; enantioselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate In tetrahydrofuran at 65℃; | 96% |
-
-
862907-06-0
tert-butyl 3-(4-fluorophenyl)-2-oxoindoline-1-carboxylate

-
-
94-36-0
dibenzoyl peroxide

-
-
1271489-24-7
tert-butyl (S)-3-(benzoyloxy)-3-(4-fluorophenyl)-2-oxoindoline-1-carboxylate

| Conditions | Yield |
|---|---|
| With Ca[(S)-VAPOL-Phosphate]2 In diethyl ether at 20℃; for 20h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 96% |
-
-
923568-89-2
tert-butyl 5-methoxy-2-oxo-3-phenylindoline-1-carboxylate

-
-
94-36-0
dibenzoyl peroxide

-
-
1271489-34-9
tert-butyl (S)-3-(benzoyloxy)-5-methoxy-2-oxo-3-phenylindoline-1-carboxylate

| Conditions | Yield |
|---|---|
| With Ca[(S)-VAPOL-Phosphate]2 In diethyl ether at 20℃; for 20h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 96% |
-
-
1175713-10-6
C19H18FNO3

-
-
94-36-0
dibenzoyl peroxide

-
-
1271489-30-5
tert-butyl (S)-3-(benzoyloxy)-7-fluoro-2-oxo-3-phenylindoline-1-carboxylate

| Conditions | Yield |
|---|---|
| With Ca[(S)-VAPOL-Phosphate]2 In diethyl ether at 20℃; for 20h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With ent-9-amino(9-deoxy)epi-hydroquinine; sodium carbonate; salicylic acid In toluene at 0℃; for 80h; optical yield given as %ee; enantioselective reaction; | 96% |
-
-
1383187-31-2
tert-butyl 5-fluoro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate

-
-
94-36-0
dibenzoyl peroxide

| Conditions | Yield |
|---|---|
| With C35H35N2O3(1+)*Br(1-); potassium carbonate In toluene at 15℃; for 12h; enantioselective reaction; | 96% |
-
-
74559-40-3
tris[tris(trimethylsilylmethyl)stannyl]neodymium*1,2-dimethoxyethane

-
-
94-36-0
dibenzoyl peroxide

-
B
-
42202-20-0
((CH3)3SiCH2)3Sn(OCOC6H5)

| Conditions | Yield |
|---|---|
| In benzene soln. of Nd-Sn complex was shaken with soln. of benzoylperoxide at 50°C for 12 h; Sn-Nd complex pptd.; elem. anal.; org. layer was sepd. by distn.; | A 95.7% B 75% |


| Conditions | Yield |
|---|---|
| With water; caesium carbonate In dichloromethane at 20℃; for 16h; | 95% |
| With pH 10.5 buffer In dichloromethane Ambient temperature; | 82% |
| With potassium carbonate In chloroform for 1h; Ambient temperature; Yield given; | |
| In dichloromethane at 20℃; pH=10.5; Inert atmosphere; Schlenk technique; | |
| With potassium hydrogenphosphate trihydrate In N,N-dimethyl-formamide at 20℃; Inert atmosphere; |
-
-
70638-09-4
4-isopropyl-2H-1,4-thiazin-3-one

-
-
94-36-0
dibenzoyl peroxide

-
-
82409-32-3
Benzoic acid 4-isopropyl-3-oxo-3,4-dihydro-2H-[1,4]thiazin-2-yl ester

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Heating; | 95% |
Benzoyl peroxide Consensus Reports
Benzoyl peroxide Standards and Recommendations
ACGIH TLV: TWA 5 mg/m3; Not Classifiable as a Human Carcinogen
DFG MAK: 5 mg/m3; Weak allergin and skin irritant
NIOSH REL: (Benzoyl Peroxide) TWA 5 mg/m3
Benzoyl peroxide Analytical Methods
Benzoyl peroxide Specification
The CAS registry number of Benzoyl peroxide is 94-36-0. The IUPAC name is benzoyl benzenecarboperoxoate. Its EINECS registry number is 202-327-6. In addition, the molecular formula is C14H10O4 and the molecular weight is 242.23. It is a kind of white powder or crystals and belongs to the classes of Industrial/Fine Chemicals; Organic Peroxide; Synthetic Organic Chemistry.
Physical properties about Benzoyl peroxide are: (1)ACD/LogP: 2.859; (2)ACD/LogD (pH 5.5): 2.86; (3)ACD/LogD (pH 7.4): 2.86; (4)ACD/BCF (pH 5.5): 87.62; (5)ACD/BCF (pH 7.4): 87.62; (6)ACD/KOC (pH 5.5): 855.31 ; (7)ACD/KOC (pH 7.4): 855.31; (8)#H bond acceptors: 4; (9)#Freely Rotating Bonds: 5; (10)Index of Refraction:1.585; (11)Molar Refractivity: 64.737 cm3; (12)Molar Volume: 193.103 cm3; (13)Polarizability: 25.664 10-24cm3; (14)Surface Tension: 49.6240005493164 dyne/cm; (15)Density: 1.254 g/cm3; (16)Flash Point: 154.239 °C; (17)Enthalpy of Vaporization: 59.427 kJ/mol; (18)Boiling Point: 349.747 °C at 760 mmHg
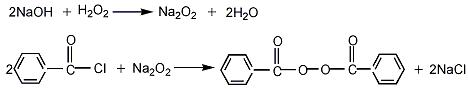
Preparation of Benzoyl peroxide: firstly, prepare the material of sodium hydroxide and oxydol. Next, drop benzoyl chloride into mixture with stirring at reaction temperature below 0 °C. Then keep the temperature about 0 °C for 2-3 hours with stirring. By means of stalling layered and drying at low temperature you can get the product.
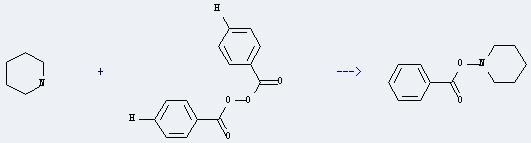
Uses of Benzoyl peroxide: it can be used to test formaldehyde and bravery sterol. And it can be used as quality improver of flour, intermediates of fine chemical products, additives of grain and oil, vulcanization agent of xylene silicone rubber and kyle F-rubber, catalyst of free radicals halogenating and radical sensitization reaction. In addition, it can react with piperidine to get 1-benzoyloxy-piperidine. This reaction will need reagent dipotassium hydrogen phosphate and solvent dimethylformamide. The reaction time is 21 hours by heating. The yield is about 60%.
When you are using this chemical, please be cautious about it as the following:
This chemical is explosive when dry. It may cause fire when contact with combustible material. And ti is irritating to eyes, respiratory system and skin and harmful in contact with skin and if swallowed. In addition, it has risk of impaired fertility. It is toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. Avoid exposure - obtain special instructions before use. It has refer to special instructions/safety data sheets. During using it, wear suitable protective clothing, gloves and eye/face protection. And keep away from combustible material. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.). In additon, this material and its container must be disposed of as hazardous waste and must be disposed of in a safe way. You shuld keep container tightly closed and avoid release to the environment.
You can still convert the following datas into molecular structure:
(1)SMILES: c1ccc(cc1)C(=O)OOC(=O)c2ccccc2
(2)InChI: InChI=1/C14H10O4/c15-13(11-7-3-1-4-8-11)17-18-14(16)12-9-5-2-6-10-12/h1-10H
(3)InChIKey: OMPJBNCRMGITSC-UHFFFAOYAV
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LD50 | skin | > 1gm/kg (1000mg/kg) | Kodak Company Reports. Vol. #900713, | |
| mouse | LD50 | intraperitoneal | 250mg/kg (250mg/kg) | International Polymer Science and Technology. Vol. 3, Pg. 93, 1976. | |
| mouse | LD50 | oral | 5700mg/kg (5700mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 32(3), Pg. 31, 1967. | |
| rat | LD50 | oral | 7710mg/kg (7710mg/kg) | LIVER: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: CYANOSIS KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION | ARCO Chemical Company Report. Vol. JUN1982, |
Related Products
- Benzoyl azide
- Benzoyl azide, 4-nitro-
- Benzoyl bromide
- Benzoyl bromide, 2-(bromomethyl)-
- Benzoyl chloride
- Benzoyl chloride, 2-(2-methoxyethoxy)- (9CI)
- Benzoyl chloride, 2-bromo-5-methoxy-
- Benzoyl chloride, 4-(methylsulfonyl)-2-nitro-
- Benzoyl chloride, 4-amino-
- Benzoyl chloride,2-(2-thienyl)-
- 943602-97-9
- 943605-97-8
- 943607-57-6
- 94361-06-5
- 94363-11-8
- 94363-18-5
- 943632-46-0
- 943650-25-7
- 943654-70-4
- 94367-20-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View