-
Name
Benzyl 2-bromoacetate
- EINECS 226-611-4
- CAS No. 5437-45-6
- Article Data65
- CAS DataBase
- Density 1.474 g/cm3
- Solubility Not miscible or difficult to mix in water.
- Melting Point 199-201 °C(Solv: water (7732-18-5))
- Formula C9H9BrO2
- Boiling Point 287.773 °C at 760 mmHg
- Molecular Weight 229.073
- Flash Point 116.485 °C
- Transport Information UN 2810
- Appearance Colorless or light yellow liquid
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Aceticacid, bromo-, benzyl ester (6CI,7CI,8CI);Acetic acid, bromo-, phenylmethylester (9CI);Benzyl bromoacetate;Bromoacetic acidbenzyl ester;Merbac 35;NSC 16114;NSC 23980;Phenylmethyl bromoacetate;Benzyl 2-bromoacetate;
- PSA 26.30000
- LogP 2.12470
Synthetic route

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene at 120℃; for 24h; | 100% |
| With toluene-4-sulfonic acid In benzene at 120℃; for 24h; Inert atmosphere; | 100% |
| With toluene-4-sulfonic acid In benzene at 120℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetonitrile at 0℃; for 0.916667h; Sealed tube; Inert atmosphere; | 98% |
| With dmap In dichloromethane at 20℃; for 2h; Inert atmosphere; | 94% |
| With 2,6-dimethylpyridine In dichloromethane at 0℃; for 2h; | 91% |

| Conditions | Yield |
|---|---|
| With 1,3-bis(2,4,6-trimethylphenyl)imidazole hydrochloride; potassium tert-butylate In benzene at 80℃; for 10h; Inert atmosphere; | 85% |
-
-
79-08-3
bromoacetic acid

-
-
100-51-6
benzyl alcohol

-
A
-
103-50-4
dibenzyl ether

-
B
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80℃; for 6h; Inert atmosphere; | A n/a B 82% |


-
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| With tetrabutylammonium hexanitratocerate(IV); carbon tetrabromide; potassium carbonate In acetonitrile at 0℃; for 2h; | 68% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; toluene unter Entfernen des entstehenden Aethanols; |
-
-
13837-45-1
1-ethoxy-1-cyclopropanol

-
-
78385-35-0
benzyloxycarbonylbromomethylenetriphenylphosphorane

-
A
-
78385-37-2
benzyl 2-bromocyclopropylideneacetate

-
B
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| In benzene for 3.5h; Yields of byproduct given; |

-
-
78385-35-0
benzyloxycarbonylbromomethylenetriphenylphosphorane

-
A
-
78385-37-2
benzyl 2-bromocyclopropylideneacetate

-
B
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| With 1-ethoxypropanol; benzoic acid In benzene for 3.5h; Yield given; |
-
-
103-50-4
dibenzyl ether

-
-
598-21-0
2-Bromoacetyl bromide

-
A
-
100-39-0
benzyl bromide

-
B
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| at 100℃; for 10h; Product distribution; |


| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium acetate trihydrate; sodium hydrogencarbonate; toluene-4-sulfonic acid; acetic acid; benzyl alcohol; aluminum nickel In ethanol; cyclohexane; water |
-
-
110-86-1
pyridine

-
-
5437-45-6
Benzyl bromoacetate

-
-
136383-19-2
N-(Benzyloxycarbonylmethyl)pyridinium bromide

| Conditions | Yield |
|---|---|
| 100% | |
| In acetone at 60℃; Inert atmosphere; |
-
-
7662-76-2
tert-butyl 2-(benzylamino)acetate

-
-
5437-45-6
Benzyl bromoacetate

-
-
185426-26-0
(Benzyl-benzyloxycarbonylmethyl-amino)-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide 1.) 0 deg C, 30 min, 2.) room temperature, 3 h; | 100% |
-
-
174799-52-1
tert-butyl {2-[benzylamino]ethyl}carbamate

-
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide 1.) 0 deg C, 30 min, 2.) room temperature, 3 h; | 100% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; | 90% |
-
-
90914-08-2
N-[N-(tert-Butyloxycarbonyl)-3-aminopropyl]benzylamine

-
-
5437-45-6
Benzyl bromoacetate

-
-
174799-95-2
[Benzyl-(3-tert-butoxycarbonylamino-propyl)-amino]-acetic acid benzyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide 1.) 0 deg C, 30 min, 2.) room temperature, 3 h; | 100% |
-
-
90914-09-3
N(1)-benzyl-N(4)-(tert-butyloxycarbonyl)-1,4-diaminobutane

-
-
5437-45-6
Benzyl bromoacetate

-
-
174799-96-3
[Benzyl-(4-tert-butoxycarbonylamino-butyl)-amino]-acetic acid benzyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide 1.) 0 deg C, 30 min, 2.) room temperature, 3 h; | 100% |
-
-
174799-53-2
(6-Benzylamino-hexyl)-carbamic acid tert-butyl ester

-
-
5437-45-6
Benzyl bromoacetate

-
-
174799-97-4
[Benzyl-(6-tert-butoxycarbonylamino-hexyl)-amino]-acetic acid benzyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide 1.) 0 deg C, 30 min, 2.) room temperature, 3 h; | 100% |
-
-
214412-65-4
(1R,5R)-6-hydroxy-7-oxo-2,6-diazabicyclo[3.2.0]heptane-2-carboxylic acid tert-butyl ester

-
-
5437-45-6
Benzyl bromoacetate

-
-
214412-66-5
(1S,5R)-6-Benzyloxycarbonylmethoxy-7-oxo-2,6-diaza-bicyclo[3.2.0]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 0℃; for 1h; | 100% |

-
-
5437-45-6
Benzyl bromoacetate

-
-
303767-15-9
2-[2-acetyl-1-adamantan-1-ylmethyl-9-(2-fluoro-benzyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carbonyl]-succinic acid 4-benzyl ester 1-tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (1R,3S)-1-[(1-adamantyl)methyl]-2-acetyl-1-(2-fluorobenzyl)-3-tert-butyloxycarbonylmethylcarbonyl-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole With sodium hydride In tetrahydrofuran at 0℃; for 0.333333h; Metallation; Stage #2: Benzyl bromoacetate In tetrahydrofuran at 20℃; for 2h; Alkylation; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| at 150℃; | 100% |
| at 50℃; for 2h; | 97% |
| at 200℃; for 1h; | 87% |
| at 80℃; for 1h; |
-
-
99-93-4
4-Hydroxyacetophenone

-
-
5437-45-6
Benzyl bromoacetate

-
-
851875-31-5
benzyl 2-(4-acetylphenoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1.5h; Williamson ether synthesis; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 75 - 80℃; for 1.5h; | 79.8% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene for 36h; Reflux; | 100% |
| With potassium carbonate In toluene for 28h; Heating; | 60% |
-
-
153086-78-3
tert-butyl {2-[2-(2-aminoethoxy)ethoxy]ethyl}carbamate

-
-
5437-45-6
Benzyl bromoacetate

-
-
875057-36-6
(benzyloxycarbonylmethyl-{2-[2-(2-tert-butoxycarbonylamino-ethoxy)-ethoxy]-ethyl}-amino)-acetic acid benzyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran; water at 20℃; for 12h; | 100% |
-
-
31252-42-3
4-benzylpyperidine

-
-
5437-45-6
Benzyl bromoacetate

-
-
438634-63-0
(4-benzylpiperidin-1-yl)acetic acid benzyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; ethyl acetate | 100% |
-
-
866464-53-1
methyl 2-(m-chlorobenzylideneamino)acetate

-
-
5437-45-6
Benzyl bromoacetate

-
-
1175019-83-6
1,3-dibenzyl-2-methyl 2-(3-chlorobenzylideneamino)propane-1,2,3-tricarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2-(m-chlorobenzylideneamino)acetate With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 1h; Inert atmosphere; Stage #2: Benzyl bromoacetate In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 100% |

-
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| Stage #1: (2R,3R)-dimethyl 2-(7,8-dihydroxy-2-phenylhexahydropyrano[3,2-d][1,3]dioxin-6-yloxy)-3-(3,4,5-tris(benzyloxy)-6-methyltetrahydro-2H-pyran-2-yloxy)succinate With di(n-butyl)tin oxide In methanol for 2h; Inert atmosphere; Reflux; Stage #2: Benzyl bromoacetate With cesium fluoride In tetrahydrofuran for 1.25h; Inert atmosphere; | 100% |
-
-
59989-18-3
5-ethynyluracil

-
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| Stage #1: Benzyl bromoacetate With copper(l) iodide; sodium azide; sodium L-ascorbate; N,N`-dimethylethylenediamine In ethanol; water at 100℃; for 1h; Stage #2: 5-ethynyluracil In ethanol; water at 100℃; for 0.5h; | 100% |
-
-
33468-67-6
2-methyl-4-(trifluoromethyl)-1H-imidazole

-
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 60℃; | 100% |

-
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; | 100% |

-
-
5437-45-6
Benzyl bromoacetate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl 4-hydroxy-[1,1'-biphenyl]-3-carboxylate With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: Benzyl bromoacetate In N,N-dimethyl-formamide at 45℃; for 2h; Williamson Ether Synthesis; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-mercaptopropionic acid With triethylamine In dichloromethane at 20℃; for 0.166667h; Stage #2: Benzyl bromoacetate In dichloromethane at -20 - 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium phosphate In acetonitrile at 20℃; for 16h; Inert atmosphere; | 99.5% |
-
-
185426-12-4
6-Benzylamino-hexanoic acid tert-butyl ester

-
-
5437-45-6
Benzyl bromoacetate

-
-
185426-28-2
6-(Benzyl-benzyloxycarbonylmethyl-amino)-hexanoic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide 1.) 0 deg C, 30 min, 2.) room temperature, 3 h; | 99.1% |
-
-
60-34-4
methylhydrazine

-
-
5437-45-6
Benzyl bromoacetate

-
-
55501-33-2
benzyl 2-(1-methylhydrazinyl)acetate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 99% |
| With triethylamine In dichloromethane 1.) 0 deg C, 0.5 h, 2.) r.t., 4 h; | 98% |

| Conditions | Yield |
|---|---|
| With indium; sodium dodecyl-sulfate at 60℃; for 0.5h; | 99% |
| With borohydride exchange resin; nickel diacetate In methanol for 1h; Ambient temperature; | 98% |
| With DMBI In tetrahydrofuran for 1h; Heating; | 94% |
-
-
350697-62-0
2-[6-[bis[(1,1-dimethylethoxy)carbonyl]amino]-1,4-dihydro-4-oxo-2-pyridinyl]-1,3-bis(1,1-dimethylethyl)ester

-
-
5437-45-6
Benzyl bromoacetate

-
-
350697-63-1
Benzyl 2-{[2,6-bis(bis(N-tert-butoxycarbonyl)amino)]-4-pyridyloxy} acetate

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In dimethyl sulfoxide at 20℃; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With air; triethyl borane In ethanol; water at 25℃; for 1.5h; | 99% |
| With air; triethyl borane; 1-ethyl-3-methylimidazolium tetrafluoroborate at 25℃; for 6h; | 86% |
-
-
852060-08-3
3-((S)-3,5-Dioxo-1,4-diaza-spiro[5.5]undec-2-yl)-propionic acid methyl ester

-
-
5437-45-6
Benzyl bromoacetate

-
-
1054660-71-7
3-((S)-4-Benzyloxycarbonylmethyl-3,5-dioxo-1,4-diaza-spiro[5.5]undec-2-yl)-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 50℃; for 2h; | 99% |
-
-
851577-43-0
5-(((tert-butoxycarbonyl)amino)methyl)-6-isobutyl-2-methyl-4-(4-methylphenyl)nicotinic acid

-
-
5437-45-6
Benzyl bromoacetate

-
-
851578-20-6
2-(benzyloxy)-2-oxoethyl 5-{[(tert-butoxycarbonyl)amino]methyl}-6-isobutyl-2-methyl-4-(4-methylphenyl)nicotinate

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; for 0.5h; | 99% |

-
-
5437-45-6
Benzyl bromoacetate

-
-
192635-89-5
phenylmethyl 2-(1,4,7,10-Tetraaza-4,7,10-tris(((tert-butyl)oxycarbonyl)methyl)cyclododecyl)-acetate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetonitrile at 100℃; for 30h; | 99% |
| With sodium hydrogencarbonate In acetonitrile at 100℃; for 30h; | 91% |
| With potassium carbonate In acetonitrile for 24h; Reflux; Inert atmosphere; | 90% |
| Stage #1: tris(tert-butyl) 2,2',2''-(1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate hydrobromide With potassium carbonate In acetonitrile for 0.5h; Stage #2: Benzyl bromoacetate In acetonitrile at 50 - 60℃; |
Benzyl 2-bromoacetate Specification
The Benzyl 2-bromoacetate, with the CAS registry number 5437-45-6, is also known as Bromoacetic acidbenzyl ester. It belongs to the product categories of Pharmaceutical Intermediates; Building Blocks; C8 to C9; Carbonyl Compounds; Chemical Synthesis; Esters; Organic Building Blocks. Its EINECS number is 226-611-4. This chemical's molecular formula is C9H9BrO2 and molecular weight is 229.07. What's more, its systematic name is Benzyl bromoacetate. This chemical is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from light.
Physical properties of Benzyl 2-bromoacetate are: (1)ACD/LogP: 2.368; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.37; (4)ACD/LogD (pH 7.4): 2.37; (5)ACD/BCF (pH 5.5): 37.13; (6)ACD/BCF (pH 7.4): 37.13; (7)ACD/KOC (pH 5.5): 462.66; (8)ACD/KOC (pH 7.4): 462.66; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.556; (14)Molar Refractivity: 49.942 cm3; (15)Molar Volume: 155.421 cm3; (16)Polarizability: 19.798×10-24cm3; (17)Surface Tension: 44.1 dyne/cm; (18)Density: 1.474 g/cm3; (19)Flash Point: 116.485 °C; (20)Enthalpy of Vaporization: 52.697 kJ/mol; (21)Boiling Point: 287.773 °C at 760 mmHg; (22)Vapour Pressure: 0.002 mmHg at 25°C.
Preparation: this chemical can be prepared by bromoacetic acid and phenylmethanol. This reaction will need reagents DCC, DMAP and solvent tetrahydrofuran with the reaction time of 16 hours. The yield is about 57%.
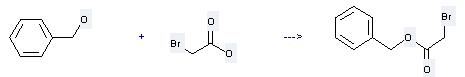
Uses of Benzyl 2-bromoacetate: it can be used to produce morpholin-4-yl-acetic acid benzyl ester at the ambient temperature. It will need solvent CH2Cl2 with the reaction time of 16 hours. The yield is about 91%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: BrCC(=O)OCc1ccccc1
(2)Std. InChI: InChI=1S/C9H9BrO2/c10-6-9(11)12-7-8-4-2-1-3-5-8/h1-5H,6-7H2
(3)Std. InChIKey: JHVLLYQQQYIWKX-UHFFFAOYSA-N
Related Products
- Benzyl (1-(aminocarbonyl)-2-hydroxypropyl)carbamate
- Benzyl (1-cyano-1-methylethyl)carbamate
- Benzyl (1R,2S)-3-chloro-2-hydroxy-1-(phenylthiomethyl)propylcarbamate
- Benzyl (2,5-dioxo-1,3-oxazolidin-4-yl)acetate
- Benzyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (2R,3S)-(1-carbamoyl-2-hydroxypropyl)carbamate
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3R)-(+)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (3-aminopropyl)carbamate
- 5437-48-9
- 5437-53-6
- 5437-54-7
- 54375-47-2
- 5437-67-2
- 54377-24-1
- 5437-98-9
- 543-80-6
- 54381-08-7
- 54381-13-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View