-
Name
Calcium cyanamide
- EINECS 205-861-8
- CAS No. 156-62-7
- Article Data378
- CAS DataBase
- Density 2.29 g/cm3
- Solubility insoluble H2O, but undergoes hydrolysis releasing acetylene and ammonia [HAW93] [MER06]
- Melting Point >300 °C(lit.)
- Formula CCaN2
- Boiling Point 258.5 °C at 760 mmHg
- Molecular Weight 80.1024
- Flash Point 42.1 °C
- Transport Information UN 1403
- Appearance Black powder or granular
- Safety 26-39-43-36/37/39-22
- Risk Codes 22-37-41-15
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Calciumcyanamide (7CI);Alzodef;Calcium cyanamide (CaCN2);Cy-L 500;Cyanamid;Lime nitrogen;Nitrogen lime;Nitrolim;Nitrolime;Perlka;
- PSA 36.15000
- LogP 0.20038
Synthetic route

| Conditions | Yield |
|---|---|
| With nitrogen In neat (no solvent) heating at 600°C for 2.5 h; color change from grey to black;; | A 100% B 100% |
| With nitrogen In neat (no solvent) heating at 600°C for 30 min; color change from grey to black;; | A 99.1% B 99.1% |
| With nitrogen In neat (no solvent) heating at 400°C for 5 h; color change from grey to black;; | A 51.8% B 51.8% |
| With nitrogen In neat (no solvent) heating at 400°C for 30 min; color change from grey to black;; | A 32.8% B 32.8% |
| In neat (no solvent) decompn.;; |
-
-
74-90-8
hydrogen cyanide

-
A
-
201230-82-2
carbon monoxide

-
B
-
1333-74-0
hydrogen

-
C
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| In neat (no solvent) equilibrium; at 800°C on right side;; pure product;; | A n/a B n/a C 99% |
| In neat (no solvent) equilibrium; at 800°C on right side;; pure product;; | A n/a B n/a C 99% |
| In neat (no solvent) | |
| In neat (no solvent) |

-
-
773837-37-9
sodium cyanide

-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With calcium chloride; iron In neat (no solvent) at 570°C, 3h;; | 82% |
| With CaCl2; iron In neat (no solvent) at 570°C, 3h;; | 82% |
| With calcium chloride In neat (no solvent) at 570°C, 30h;; | 21.3% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) 3 h heating of Ca3N2 and graphite at 1180°C; quenching;; | A 6.5% B 68% |
| In neat (no solvent) equil.; 3 h heating at 1180°C; quenching with cold water;; | A 6.5% B 68% |
| In neat (no solvent) 3 h heating of Ca3N2 and graphite at 1180°C; quenching;; | A 6.5% B 68% |
| In neat (no solvent) equil.; 5 h heating at 1060°C; quenching with cold water;; | A 6.7% B 58% |


-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CO2; 15 h heating at 410°C in vac.;; | 23.7% |
| In neat (no solvent) byproducts: CO2; 15 h heating at 375-380°C;; | 8.5% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 1200°C; equilibrium depends on temp.; divariante equilibrium with N2 and C phases and soln. of CaC2 in CaCN2 or CaO;; | A 21% B n/a |
| In neat (no solvent) conducting process in presence of CaF2; kiln described;; | |
| In neat (no solvent) Frank Caro apparatus described;; |


| Conditions | Yield |
|---|---|
| at 800℃; Waermetoenung; | |
| at 800℃; |


-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With nitrogen zur technischen Gewinnung; | |
| With nitrogen Einfluss der Koernung des verwendeten Calciumcarbids und der Menge der Katalysatoren (CaF2,CaCl2,Aktivkohle,Thomasschlacke,CaCN2) auf die Geschwindigkeit der Bildung; | |
| With nitrogen zur technischen Gewinnung; |

-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With carbon dioxide; ammonia at 600 - 800℃; | |
| With carbon monoxide; ammonia at 600 - 800℃; | |
| With ammonia at 600 - 800℃; |

| Conditions | Yield |
|---|---|
| With ammonia at 750℃; | |
| With ammonia at 750℃; Wiederverwendung der Restgase; | |
| With ammonia at 750℃; | |
| With ammonia at 750℃; vorhergehendes teilweises oder vollstaendiges Brennen der Carbonate; |

-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With calcium nitride; nitrogen at 800 - 1100℃; |

-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With aluminum oxide; nitrogen; ammonia; pyrographite; calcium oxide at 750 - 850℃; | |
| With ammonia; water; calcium oxide |

| Conditions | Yield |
|---|---|
| With aluminum oxide; nitrogen; ammonia; pyrographite at 750 - 850℃; | |
| With ammonia; water |

| Conditions | Yield |
|---|---|
| at 600 - 800℃; |

| Conditions | Yield |
|---|---|
| at 600 - 800℃; |


-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With nitrogen bei Dunkelrotglut bis Weissglut; | |
| With nitrogen at 1000 - 1100℃; | |
| With nitrogen bei Dunkelrotglut bis Weissglut; |

-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With nitrogen technische Darstellung; | |
| With nitrogen at 1200 - 1300℃; Gleichgewicht; | |
| With nitrogen |

| Conditions | Yield |
|---|---|
| at 900℃; |

-
-
156-62-7
calcium cyanamide

| Conditions | Yield |
|---|---|
| With ammonia | |
| With carbon monoxide; ammonia at 630 - 700℃; |

| Conditions | Yield |
|---|---|
| at 750 - 850℃; |
-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
75-77-4
chloro-trimethyl-silane

-
-
156-62-7
calcium cyanamide

-
B
-
1000-70-0
bis(trimethylsilyl)carbodi-imide

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide HMPTA added to suspn. of CaCN2 in Me3SiCl (stirring); heated to 60°C; heated gradually (120°C, 3 h); cooled; hexane added; ppt. filtered off; dried (vac., 90°C); liquid phase fractionated; elem.anal.; | A 97% B n/a |


| Conditions | Yield |
|---|---|
| With pyrographite In neat (no solvent) heating to 1300°C, fast cooling;; | 95.5% |
| With pyrographite In neat (no solvent) heating to 1300°C, fast cooling;; | 95.5% |
| With pyrographite In neat (no solvent) at 900°C;; | 10% |

| Conditions | Yield |
|---|---|
| With aluminum(III) sulfate In water | 95% |
| With Al2(SO4)3 In water | 95% |
| With sulfur dioxide byproducts: calcium sulfite; pptn. of Ca with SO2; evapn.; | 90% |

| Conditions | Yield |
|---|---|
| With carbon dioxide 40°C; | 92% |
| With sulfuric acid | |
| With nitric acid |
-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
156-62-7
calcium cyanamide

-
-
1067-10-3
Bromotriethylgermanium

-
B
-
32673-14-6
bis(triethylgermyl)carbodiimide

| Conditions | Yield |
|---|---|
| In xylene heated (1 h, 150°C); cooled; ppt. filtered off; stirred (warm H2O); filtered off; H2O evapd.; dried (vac., 70°C); xylene filtrate fractionated; elem.anal.; | A 90.5% B 77.3% |


| Conditions | Yield |
|---|---|
| With carbon dioxide In methanol 40°C, 3 h; filtration, evapn. of solvent at 11 Torr; | 90% |
| With carbon dioxide In not given byproducts: CaCO3; leading of CO2, filtration of CaCO3, acidifying with acetic acid to pH=6; evapn. at 40°C and 10-20 Torr, cooling down to ambient temp., after concentration cooling down to 5-10°C, drying in vac.; dissolving in ether, filtration, evapn. of ether or pptn. with benzene, distillation at 85-87°C and 0.5 Torr; | |
| With carbon dioxide byproducts: CaCO3; 30-32°C; |
-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
1112-48-7
triethyl-bromo-silane

-
-
156-62-7
calcium cyanamide

-
B
-
7414-77-9
bis(triethylsilyl)carbodiimide

| Conditions | Yield |
|---|---|
| In xylene heated (150°C, 1 h); cooled to 120°C; liquid decanted; fractionated (vac.); ppt. washed (hexane); dried (vac.); elem.anal.; | A 89.4% B 81.6% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 1300 - 1450°C;; | 88% |
| In neat (no solvent) at 1300 - 1450°C;; | 88% |
| In neat (no solvent) at 1300 - 1450°C;; | 88% |
| In neat (no solvent) addn. of metallic Na supplying raw material to CaCN2 on cyanide bath at 1400°C;; | |
| In neat (no solvent) addn. of metallic Na supplying raw material to CaCN2 on cyanide bath at 1400°C;; |

| Conditions | Yield |
|---|---|
| With phosphoric acid; sulfuric acid | 86% |
| phosphoric acid | 75% |
| sulfuric acid |

| Conditions | Yield |
|---|---|
| Stage #1: calcium cyanamide; methyl chloroformate In water at 38 - 41℃; for 1h; Stage #2: 2',3,4-triaminodiphenyl ether In acetic acid; isopropyl alcohol for 6h; Heating; | 84% |

-
-
6264-66-0
1,2-diamino-4-(4-aminophenoxy)benzene

-
-
156-62-7
calcium cyanamide

-
-
79-22-1
methyl chloroformate

-
-
56073-92-8
methyl (5-(4-aminophenoxy)-1H-benzimidazol-2-yl)carbamate

| Conditions | Yield |
|---|---|
| Stage #1: calcium cyanamide; methyl chloroformate In water at 38 - 41℃; for 1h; Stage #2: 1,2-diamino-4-(4-aminophenoxy)benzene In acetic acid; isopropyl alcohol for 6h; Heating; | 83% |


| Conditions | Yield |
|---|---|
| In water at 85 - 90℃; for 1.5h; | 82% |

-
-
125159-34-4
3,4,4'-triaminodiphenyl sulfide

-
-
156-62-7
calcium cyanamide

-
-
79-22-1
methyl chloroformate

-
-
56073-96-2
methyl N-[5(6)-(4-aminophenylsulfanyl)benzimidazol-2-yl]carbamate

| Conditions | Yield |
|---|---|
| Stage #1: calcium cyanamide; methyl chloroformate In water at 38 - 41℃; for 1h; Stage #2: 3,4,4'-triaminodiphenyl sulfide In water; acetic acid; isopropyl alcohol for 6h; Heating; | 82% |


| Conditions | Yield |
|---|---|
| With hydrogen wt ratio = 1:1; at 1400-1750°C, in 93% N2, 7% H2 atmosphere; | 82% |

-
-
75-15-0
carbon disulfide

-
-
156-62-7
calcium cyanamide

-
-
77-78-1
dimethyl sulfate

-
-
10191-60-3
dimethyl N-cyanodithioiminocarbonate

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide; calcium cyanamide With sodium carbonate In water at 40 - 45℃; for 5h; Stage #2: dimethyl sulfate at 0 - 20℃; for 2h; | 82% |


| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide; xylene for 1h; Heating; | 81.6% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
156-62-7
calcium cyanamide

-
-
1000-70-0
bis(trimethylsilyl)carbodi-imide

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide for 3h; Heating; | 75.7% |

| Conditions | Yield |
|---|---|
| In methanol; water H2O-MeOH: fourfold excess; stoichiometric; washed (EtOH); dried (100°C); elem. anal.; | 75% |
| In neat (no solvent) stoichiometric ratio; washed (EtOH); dried (100°C); elem. anal.; |
-
-
156-62-7
calcium cyanamide

-
-
150-13-0
4-amino-benzoic acid

-
-
42823-46-1
4-guanidinobenzoic acid hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 80℃; for 3h; Cooling with ice; | 45.8% |
-
-
680-31-9
N,N,N,N,N,N-hexamethylphosphoric triamide

-
-
1461-22-9
tributyltin chloride

-
-
156-62-7
calcium cyanamide

-
B
-
34885-41-1
bis(tributylstannyl)carbodiimide

| Conditions | Yield |
|---|---|
| In toluene CaCN2 in HMPTA added to Bu3SnCl; heated to 40°C; toluene added; boiled (3 h); cooled; ppt. filtered; Bu3SnCl removed; benzene added; filtred off; filtrate chromd. (Al2O3); solvent evapd.; elem.anal.; | A n/a B 38.4% |


| Conditions | Yield |
|---|---|
| With air; copper(II) oxide; copper(II) carbonate; nickel(II) carbonate In neat (no solvent) storage of thin layer of mixt. of lime nitrogen, soda lime and CuO on air for few days; addn. of catalyst, heating in dry stream of air at 400°C, until oxidn. of C is complete; heating up to 3 h at 450°C; acceleration by min. of H2O vapor;; | 38% |
-
-
426-67-5
triisopropylsilyl fluoride

-
-
156-62-7
calcium cyanamide

-
-
86071-46-7
Bis(triisopropylsilyl)-carbodiimid

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide; xylene at 145℃; for 4h; | 31.2% |

| Conditions | Yield |
|---|---|
| cerium nitride 800-1000°C; | 25% |
| aluminium nitride 800-1000°C; | 25% |
| magnesium nitride 800-1000°C; | 25% |

Calcium cyanamide Chemical Properties
Product Name: Calcium cyanamide (CAS NO.156-62-7)
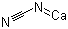
Molecular Formula: CCaN2
Molar mass: 80.10 g/mol
EINECS: 205-861-8
Density: 2.29 g/cm3
Flash Point: 42.1 °C
Melting point: 1340 °C
Water solubility: Reacts
Boiling Point: 1150-1200 °C (sublim.)
Appearance: White solid (Often gray or black from impurities)
Solubility: soluble in water, alcohol, acetone
H-Bond Donor: 1
H-Bond Acceptor: 2
Structure Descriptors of Calcium cyanamide (CAS NO.156-62-7):
IUPAC Name: calcium cyanamide
Canonical SMILES: C(#N)N.[Ca+2]
InChI: InChI=1S/CH2N2.Ca/c2-1-3;/h2H2;/q;+2
InChIKey: STRTXDFFNXSZQB-UHFFFAOYSA-N
Product Categories: Pharmaceutical Intermediates; Inorganics
Calcium cyanamide Uses
The main use of Calcium cyanamide (CAS NO.156-62-7) is in agriculture as a fertilizer. In contact with water it decomposes and liberates ammonia:
CaCN2 + 3 H2O → 2 NH3 + CaCO3
It was used in the preparation of melamine and calcium cyanide.And it can also be used to produce sodium cyanide by fusing with sodium carbonate, which was used in cyanide process in gold mining:
CaCN2 + Na2CO3 → 2 NaCN + CaO + O2
Calcium cyanamide Production
Calcium cyanamide (CAS NO.156-62-7) is formed when calcium carbide reacts with nitrogen.
CaC2 + N2 → CaCN2 + C
The reaction takes place in large steel chambers. An electric carbon element heats the reactants to red heat. Nitrogen is pressurised at 2 atmospheres.
Calcium cyanamide Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | LDLo | oral | 571mg/kg (571mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 149, 1969. | |
| mouse | LD50 | intraperitoneal | 100mg/kg (100mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | intravenous | 282mg/kg (282mg/kg) | Drugs in Japan Vol. 6, Pg. 304, 1982. | |
| mouse | LD50 | oral | 334mg/kg (334mg/kg) | Drugs in Japan Vol. 6, Pg. 304, 1982. | |
| rabbit | LD50 | oral | 1400mg/kg (1400mg/kg) | Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 174, 1966. | |
| rabbit | LD50 | skin | 590mg/kg (590mg/kg) | "Kirk-Othmer Encyclopedia of Chemical Technology," 3rd ed., Grayson, M., and D. Eckroth, eds. New York, John Wiley & Sons, Inc., 1978Vol. 7, Pg. 291, 1979. | |
| rat | LC50 | inhalation | > 150mg/m3/4H (150mg/m3) | "Agrochemicals Handbook," with updates, Hartley, D., and H. Kidd, eds., Nottingham, Royal Soc of Chemistry, 1983-86Vol. A053, Pg. 1984, | |
| rat | LD50 | intravenous | 125mg/kg (125mg/kg) | Drugs in Japan Vol. 6, Pg. 304, 1982. | |
| rat | LD50 | oral | 158mg/kg (158mg/kg) | Drugs in Japan Vol. 6, Pg. 304, 1982. | |
| rat | LD50 | unreported | 1gm/kg (1000mg/kg) | Guide to the Chemicals Used in Crop Protection. Vol. 6, Pg. 73, 1973. |
Calcium cyanamide Consensus Reports
NCI Carcinogenesis Bioassay (feed); No Evidence: mouse, rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-163 ,1979. . Community Right-To-Know List. Reported in EPA TSCA Inventory.
Calcium cyanamide Safety Profile
Poison by ingestion, inhalation, skin contact, intravenous, and intraperitoneal routes. Moderately toxic to humans by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. The fatal dose, by ingestion, is probably around 20 to 30 g for an adult. It does not have a cyanide effect. Calcium cyanamide is not believed to have a cumulative action. Flammable. Reaction with water forms the explosive acetylene gas. When heated to decomposition it emits toxic fumes of NOx and CN−. See also CALCIUM COMPOUNDS, AMIDES, and CYANIDE.
Safety Information of Calcium cyanamide (CAS NO.156-62-7):
Hazard Note: Harmful Irritant
Hazard Codes: Xn
Risk Statements:
22: Harmful if swallowed
37: Irritating to the respiratory system
41: Risk of serious damage to eyes
Safety Statements:
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
Calcium cyanamide Standards and Recommendations
OSHA PEL: TWA 0.5 mg/m3
ACGIH TLV: TWA 0.5 mg/m3; Not Classifiable as a Human Carcinogen
DFG MAK: 1 mg/m3
DOT Classification: 4.3; Label: Dangerous When Wet
Calcium cyanamide Specification
Calcium cyanamide (CAS NO.156-62-7) also can be called Cyanamide calcium salt , UN 1403 , Lime Nitrogen , Nitrolime .It is a calcium compound used as fertiliser, first synthesized in 1898 by Adolph Frank and Nikodem Caro.It is commercially known as Nitrolim.It crystalizes in hexagonal crystal system with space group R3m and lattice constants a = 3.67, c = 14.85 (.10-1 nm).
Related Products
- Calcium
- Calcium valproate
- Calcium (1)-bis(2-hydroxy-4-methylvalerate)
- Calcium (2S)-2-[(4-chlorobenzoyl)amino]-3-(1H-indol-3-yl)propanoate
- Calcium (S)-3-methyl-2-oxovalerate
- Calcium 2,4-dichlorophenolate
- Calcium 2-ethylhexanoate
- Calcium 2-hydroxypropanoate
- Calcium 2-methoxyethoxide
- Calcium 2-oxoglutarate
- 15663-27-1
- 156635-87-9
- 156639-36-0
- 15664-19-4
- 156641-98-4
- 156642-03-4
- 15664-29-6
- 156647-96-0
- 156648-40-7
- 15666-97-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View