-
Name
Chloroacetone
- EINECS 201-161-1
- CAS No. 78-95-5
- Article Data142
- CAS DataBase
- Density 1.162 g/cm3
- Solubility 124 g/L (20 °C ) in water
- Melting Point -44.5 °C
- Formula C3H5ClO
- Boiling Point 120 °C at 760 mmHg
- Molecular Weight 92.5251
- Flash Point 27.8 °C
- Transport Information UN 1695 6.1/PG 1
- Appearance colourless to dark yellow liquid
- Safety 16-23-36/37/39-45-60-61-38-28A-26
- Risk Codes 10-24/25-26-36/37/38-50/53
-
Molecular Structure
-
Hazard Symbols
 T,
T,  F,
F,  Xi,
Xi,  T+,
T+,  N
N
- Synonyms 1-chloropropan-2-one;
- PSA 17.07000
- LogP 0.81420
Synthetic route

| Conditions | Yield |
|---|---|
| With chlorine; calcium carbonate In water at 110℃; Reagent/catalyst; Temperature; | 95.6% |
| With methanol; sulfuryl dichloride In dichloromethane | 85% |
| With sulfuryl dichloride |
-
-
35952-61-5
(C4H9)3SnOCH(CH2Cl)2

-
A
-
1461-22-9
tributyltin chloride

-
B
-
78-95-5
chloroacetone

-
C
-
106-89-8
epichlorohydrin

| Conditions | Yield |
|---|---|
| 63% decompn. at 210°C (1 h); | A n/a B 5% C 95% |


| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid; boron trifluoride diethyl etherate In acetic acid for 1h; Heating; Yields of byproduct given; | A n/a B 88% |
| With water; chlorine; calcium carbonate |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 3h; Heating; | 85% |

| Conditions | Yield |
|---|---|
| With HT4-c823 In water at 149.84℃; under 750.075 Torr; for 0.5h; Catalytic behavior; Reagent/catalyst; Temperature; Flow reactor; chemoselective reaction; | A 18.9% B 80.5% |

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane for 1h; Ambient temperature; | A 50% B 14% |
-
-
2684-62-0
diazoacetone

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
78-95-5
chloroacetone

-
B
-
177084-70-7
2-diazo-3-oxobutanal

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In dichloromethane for 1h; Ambient temperature; | A 50% B 14% |

-
-
2684-62-0
diazoacetone

-
B
-
116-09-6
hydroxy-2-propanone

-
C
-
78-95-5
chloroacetone

-
D
-
76089-31-1
1,1'-oxydi(propan-2-one)

| Conditions | Yield |
|---|---|
| With hydrogenchloride; gallium(III) trichloride; water In dichloromethane at 20℃; for 0.0833333h; | A 34% B 8% C 20% D 31% |

-
-
173063-66-6
cis/trans-3,5-Bis-(chloromethyl)-3-fluoro-5-methyl-1,2,4-trioxolane

-
A
-
78-95-5
chloroacetone

-
B
-
79-04-9
chloroacetyl chloride

| Conditions | Yield |
|---|---|
| With triphenylphosphine In chloroform-d1 | A 28 % Spectr. B 23 % Spectr. C 33% |

-
-
115290-60-3
{Pd2(μ-H)(COCH3)2(μ-dppm)2}BPh4

-
-
98194-67-3
Pd2(CH3)2(Cl)2(μdppm)2

-
B
-
75-07-0
acetaldehyde

-
C
-
78-95-5
chloroacetone

| Conditions | Yield |
|---|---|
| In dichloromethane warming of {Pd2(μ-H)(COCH3)2(dppm)2}BPh4 in CH2Cl2 (23°C, 24 h); | A 32% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With chlorine at 0 - 10℃; Kochen des Reaktionsprodukts mit Wasser; |

| Conditions | Yield |
|---|---|
| With sulfuric acid Destillieren der Loesung mit Wasser; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; diethyl ether | |
| Stage #1: diazoacetone With ethylaluminum dichloride In hexane; dichloromethane at 20℃; for 0.0166667h; Stage #2: With methanol In hexane; dichloromethane |

| Conditions | Yield |
|---|---|
| With water; chlorine |
-
-
67-66-3
chloroform

-
-
29843-58-1
1,4-dichloro-2-methyl-2-butene

-
A
-
107-20-0
2-chloroethanal

-
B
-
78-95-5
chloroacetone

| Conditions | Yield |
|---|---|
| beim Ozonisierung und Behandeln des Ozonids mit Wasser bei 75-80grad; |


| Conditions | Yield |
|---|---|
| bei der Ozonspaltung; |

| Conditions | Yield |
|---|---|
| beim Destillieren unter gewoehnlichem Druck; |

| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| at -20℃; |


| Conditions | Yield |
|---|---|
| With N-oxo-N-nitrosoamine at 280℃; under 367754 Torr; |

| Conditions | Yield |
|---|---|
| With chromic acid | |
| With ruthenium(IV) oxide; sodium chloride In water; ethyl acetate electrooxidation; | |
| With sodium hydroxide; N-bromoacetamide In water at 24.9℃; Mechanism; Kinetics; Thermodynamic data; var. of temp. (Tab. VI); ΔH(excit.), ΔS(excit.), ΔF(excit.); |
-
-
75-56-9, 16033-71-9
methyloxirane

-
-
78-95-5
chloroacetone

| Conditions | Yield |
|---|---|
| With chlorine at 0℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride 1.) ether, 2 h, -20 to 0 deg C, 2.) -20 deg C to room temp.; Multistep reaction; |

| Conditions | Yield |
|---|---|
| for 48h; |
-
-
96-23-1
1,3-Dichloro-2-propanol

-
A
-
616-23-9
2,3-Dichloro-1-propanol

-
B
-
96-18-4
1,2,3-trichloropropane

-
C
-
23916-48-5
1-chloro-3-(2-chloro-1-chloromethyl-ethoxy)-propan-2-ol

-
D
-
78-95-5
chloroacetone

| Conditions | Yield |
|---|---|
| at 180℃; Product distribution; also in the presence of 1.5 percent HCl or 5percent epichlorhydrin; |


| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 1h; | 100% |
| With potassium carbonate In tetrahydrofuran |
-
-
2687-43-6
O-benzylhydoxylamine hydrochloride

-
-
78-95-5
chloroacetone

-
-
83200-26-4
1-chloropropan-2-one O-benzyloxime

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 20℃; for 5h; | 100% |
| With sodium hydroxide In methanol 2.) room temp., 5 h; | 91% |
-
-
123979-29-3
2,6-dimethyl-1,5-dihydroxynaphthalene

-
-
78-95-5
chloroacetone

-
-
137517-19-2
2,6-Dimethyl-1,5-bis(2-oxopropoxy)naphthalene

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Ambient temperature; | 100% |
-
-
137517-25-0
2,6-Dipropyl-1,5-naphthalenediol

-
-
78-95-5
chloroacetone

-
-
137517-22-7
2,6-Dipropyl-1,5-bis(2-oxopropoxy)naphthalene

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Ambient temperature; | 100% |
-
-
132483-78-4
Diethyl 2-methyl-2-(4-thiocarbamoylphenyl)malonate

-
-
78-95-5
chloroacetone

-
-
138569-11-6
Diethyl 2-methyl-2-<4-(4-methylthiozol-2-yl)phenyl>malonate

| Conditions | Yield |
|---|---|
| In benzene for 3.25h; Heating; | 100% |
-
-
78-95-5
chloroacetone

-
-
18156-75-7
1-trimethylsilylpyrazole

| Conditions | Yield |
|---|---|
| at 80℃; for 1h; | 100% |
-
-
78-95-5
chloroacetone

-
-
18293-54-4
1-trimethylsilyl-1,2,4-triazole

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; | 100% |
-
-
78-95-5
chloroacetone

-
-
43183-36-4
1-(trimethylsilyl)-1H-benzotriazole

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; | 100% |
-
-
78-95-5
chloroacetone

-
-
15264-63-8
5-(4-pyridinyl)-1,3,4-oxadiazole-2-thiol

-
-
81555-96-6
1-(5-Pyridin-4-yl-[1,3,4]oxadiazol-2-ylsulfanyl)-propan-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 12h; Ambient temperature; | 100% |
-
-
110766-90-0
8-Chloro-2-methyl-2,3-dihydro-5H-benzo[b][1,4]thiazepin-4-one

-
-
78-95-5
chloroacetone

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide In tetrahydrofuran Ambient temperature; | 100% |
-
-
78-95-5
chloroacetone

-
-
216776-76-0
6-Fluoro-4-methyl-2-phenyl-thieno[2,3-d]pyrimidin-5-ylamine

-
-
115073-27-3
6-acetyl-5-amino-4-methyl-2-phenylthieno[2,3-d]pyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate | 100% |

| Conditions | Yield |
|---|---|
| at 60℃; for 12h; Solid phase reaction; substitution; | 100% |
-
-
78-95-5
chloroacetone

-
-
239131-42-1
5-(2-chlorophenyl)-3-mercapto-2-cyclohexen-1-one

-
-
239131-43-2
5-(2-chlorophenyl)-3-[(2-oxopropyl)thio]-2-cyclohexen-1-one

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20℃; for 5h; | 100% |
| With sodium ethanolate In ethanol at 20℃; for 5h; |

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide | 100% |
| With sodium hydride In dimethyl sulfoxide at 20℃; | 23% |
-
-
496-69-5
2-bromo-4-fluoro-phenol

-
-
78-95-5
chloroacetone

-
-
286836-12-2
1-(2-bromo-4-fluorophenoxy)propan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In tetrahydrofuran for 4h; Heating / reflux; | 100% |
| With potassium carbonate; potassium iodide In tetrahydrofuran for 4h; Heating / reflux; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; | 59% |
| With potassium carbonate; potassium iodide In acetone at 55℃; for 2h; | |
| With potassium iodide; potassium carbonate In tetrahydrofuran; ethyl acetate | 2.7 gm (100%) |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone at 0 - 20℃; for 1h; Heating / reflux; | 100% |
-
-
872707-11-4
N-[4-({[tert-butyl(dimethyl)silyl]oxy}methyl)pyridin-2-yl]thiourea

-
-
78-95-5
chloroacetone

-
-
872707-12-5
{2-[(4-methyl-1,3-thiazol-2-yl)amino]pyridin-4-yl}methanol

| Conditions | Yield |
|---|---|
| In ethanol for 16h; Heating / reflux; | 100% |
| In ethanol for 16h; Heating / reflux; | 100% |
| In ethanol for 16h; Heating / reflux; Under nitrogen; | 100% |
-
-
1059686-59-7
2-Cyclopropylamino-4,6-difluoro-benzoic acid

-
-
78-95-5
chloroacetone

-
-
1059686-60-0
2-Cyclopropylamino-4,6-difluoro-benzoic acid 2-oxo-propyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-Cyclopropylamino-4,6-difluoro-benzoic acid With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 1h; Stage #2: chloroacetone In N,N-dimethyl-formamide at 20 - 55℃; for 1.5h; | 100% |
-
-
953045-29-9
1-(5-bromo-3-(phenylthio)pyridin-2-yl)thiourea

-
-
78-95-5
chloroacetone

-
-
953040-73-8
N-(5-bromo-3-(phenylthio)pyridin-2-yl)-4-methylthiazol-2-amine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 70℃; for 6h; | 100% |
-
-
1189115-86-3
tert-butyl (S)-1-amino-3-(1H-indol-3-yl)-1-thioxopropan-2-yl carbamate

-
-
78-95-5
chloroacetone

-
-
1189115-87-4
tert-butyl [(S)-1-(4-hydroxy-4-methyl-4,5-dihydro-thiazol-2-yl)-2-(1H-indol-3-yl)-ethyl]-carbamate

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In 1,2-dimethoxyethane at 45 - 70℃; | 100% |
-
-
1186033-44-2
C9H9ClOS

-
-
78-95-5
chloroacetone

-
-
66490-34-4
1-(4-chloro-1H-benzo[b]thiophen-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With calcium oxide In acetone at 59℃; | 100% |
| With calcium oxide for 3h; Reflux; |
-
-
53606-33-0
2-(ethylsulfanyl)benzenecarbaldehyde

-
-
78-95-5
chloroacetone

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With calcium oxide In acetone at 59℃; | 100% |
| With calcium oxide for 3h; Reflux; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60℃; for 20h; | 100% |
| With sodium hydrogencarbonate In toluene at 110 - 120℃; for 3h; Inert atmosphere; | 22.2% |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide In water at 0 - 5℃; Inert atmosphere; | 100% |
-
-
78-95-5
chloroacetone

-
-
188115-27-7
(S)-11-thioxo-2,3,11,11a-tetrahydro-1H-benzo[e]-pyrrolo[1,2-a][1,4]diazepin-5(10H)-one

-
-
1271450-06-6
C15H16N2O2S

| Conditions | Yield |
|---|---|
| Stage #1: (S)-11-thioxo-2,3,11,11a-tetrahydro-1H-benzo[e]-pyrrolo[1,2-a][1,4]diazepin-5(10H)-one With sodium hydride In dimethyl sulfoxide at 20℃; for 0.5h; Inert atmosphere; Stage #2: chloroacetone In dimethyl sulfoxide at 20℃; for 0.666667h; Inert atmosphere; | 100% |
-
-
1152617-24-7
2-amino-3-chloro-4-iodopyridine

-
-
78-95-5
chloroacetone

-
-
1416551-60-4
8-chloro-7-iodo-2-methylimidazo[1,2-a]pyridine

| Conditions | Yield |
|---|---|
| In ethanol at 65℃; | 100% |
-
-
576-23-8
2,3-dimethylbromobenzene

-
-
78-95-5
chloroacetone

-
-
42432-45-1
2-(2,3-dimethylphenyl)-2-methyloxirane

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dimethylbromobenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: chloroacetone In tetrahydrofuran; hexane; toluene at -78 - 20℃; for 1.33333h; Product distribution / selectivity; | 100% |
| Stage #1: 2,3-dimethylbromobenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: chloroacetone In tetrahydrofuran; hexane; toluene at -78 - 20℃; Product distribution / selectivity; | 100% |
-
-
59108-90-6
1H-indole-3-carbothioamide

-
-
78-95-5
chloroacetone

-
-
93588-10-4
4-methyl-2-(3-indolyl)thiazole

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; for 1.5h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; acetonitrile at 20℃; for 8h; Reflux; | 100% |
| With potassium carbonate In dichloromethane; acetonitrile for 8h; Reflux; | 100% |
| With potassium carbonate In dichloromethane; acetonitrile for 8h; Reflux; |
Chloroacetone Consensus Reports
Chloroacetone Standards and Recommendations
DOT Classification: 6.1; Label: Poison (UN 1695); DOT Class: Forbidden
Chloroacetone Specification
The Chloroacetone, also known as 2-Propanone, 1-chloro-, is an chemical compound with the formula C3H5ClO. It belongs to the product categories of Pharmaceutical Intermediates; Organics. Its EINECS registry number is 201-161-1. With the CAS registry number 78-95-5, its IUPAC name is 1-chloropropan-2-one. What's more, this chemical is a colourless to dark yellow liquid.
Physical properties of Chloroacetone: (1)ACD/LogP: 0.43; (2)ACD/LogD (pH 5.5): 0.43; (3)ACD/LogD (pH 7.4): 0.43; (4)ACD/BCF (pH 5.5): 1.24; (5)ACD/BCF (pH 7.4): 1.24; (6)ACD/KOC (pH 5.5): 40.57; (7)ACD/KOC (pH 7.4): 40.57; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 1; (10)Index of Refraction: 1.396; (11)Molar Refractivity: 20.82 cm3; (12)Molar Volume: 86.5 cm3; (13)Surface Tension: 25.7 dyne/cm; (14)Density: 1.068 g/cm3; (15)Flash Point: 27.8 °C; (16)Enthalpy of Vaporization: 35.82 kJ/mol; (17)Boiling Point: 120 °C at 760 mmHg; (18)Vapour Pressure: 15.5 mmHg at 25°C.
Preparation: this reaction will use acetone as raw materials. It will need chlorine which should be decompose produced by potassium chlorate and hydrogen chloride.
3CH3COCH3 + KClO3 + 3HCl → 3CH3COCH2Cl + KCl + 3H2O
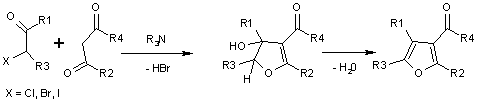
Uses: Chloroacetone is used to make dye couplers for colour photography, and is an intermediate in chemical manufacturing. It is also used in the Feist-Benary synthesis of furans.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(=O)CCl
(2)InChI: InChI=1S/C3H5ClO/c1-3(5)2-4/h2H2,1H3
(3)InChIKey: BULLHNJGPPOUOX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | skin | 100uL/kg (0.1mL/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| human | LCLo | inhalation | 605ppm/10M (605ppm) | National Technical Information Service. Vol. PB214-270, | |
| mouse | LD50 | intraperitoneal | 92mg/kg (92mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: TREMOR | Oyo Yakuri. Pharmacometrics. Vol. 33, Pg. 695, 1987. |
| mouse | LD50 | oral | 127mg/kg (127mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): HAIR: OTHER BEHAVIORAL: ATAXIA | American Industrial Hygiene Association Journal. Vol. 47, Pg. 375, 1986. |
| rabbit | LD50 | skin | 141mg/kg (141mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA SKIN AND APPENDAGES (SKIN): HAIR: OTHER | American Industrial Hygiene Association Journal. Vol. 47, Pg. 375, 1986. |
| rat | LC50 | inhalation | 262ppm/1H (262ppm) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): HAIR: OTHER BEHAVIORAL: ATAXIA | American Industrial Hygiene Association Journal. Vol. 47, Pg. 375, 1986. |
| rat | LD50 | intraperitoneal | 80mg/kg (80mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: TREMOR | Oyo Yakuri. Pharmacometrics. Vol. 33, Pg. 695, 1987. |
| rat | LD50 | oral | 100mg/kg (100mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA SKIN AND APPENDAGES (SKIN): HAIR: OTHER | American Industrial Hygiene Association Journal. Vol. 47, Pg. 375, 1986. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View