-
Name
Chloropheniramine-d4
- EINECS
- CAS No. 132-22-9
- Article Data20
- CAS DataBase
- Density 1.107g/cm3
- Solubility 5.496g/L(37.5 oC)
- Melting Point 25°C
- Formula C16H19 Cl N2
- Boiling Point 379°Cat760mmHg
- Molecular Weight 274.793
- Flash Point 183°C
- Transport Information
- Appearance
- Safety Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. When heated to decomposition it emits toxic vapors of NOx and Cl−.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Pyridine,2-[p-chloro-a-[2-(dimethylamino)ethyl]benzyl]-(8CI); (?à)-Chloropheniramine; (?à)-Chlorpheniramine;1-(p-Chlorophenyl)-1-(2-pyridyl)-3-dimethylaminopropane; 2-[p-Chloro-a-[2-(dimethylamino)ethyl]benzyl]pyridine;3-(p-Chlorophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine; 4-Chloropheniramine;Allergican; Chloropheniramine; Chlorophenylpyridamine; Chloroprophenpyridamine;Chlorphenamine; Chlorpheniramine; Chlorprophenpyridamine; Haynon;RS-Chlorpheniramine;dl-1-(p-Chlorophenyl)-1-(2-pyridyl)-3-(dimethylamino)propane; g-(4-Chlorophenyl)-g-(2-pyridyl)propyldimethylamine
- PSA 16.13000
- LogP 3.81860
Synthetic route
-
-
55486-47-0
3-(p-chlorophenyl)-3-(2-pyridyl)propanal

-
-
124-40-3
dimethyl amine

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In toluene at 120℃; under 38000 Torr; for 12h; catalytic reductive amination; | 100% |
-
-
65676-21-3
chloropheniramine nitrile

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 160℃; for 4h; | 95% |
-
-
73486-85-8
3-(4-chlorophenyl)-3-pyridin-2-yl-propan-1-ol

-
-
124-40-3
dimethyl amine

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; bis[2-(diphenylphosphino)phenyl] ether In toluene at 20℃; Inert atmosphere; Reflux; | 81% |
-
-
73775-50-5
2-(4-chloro-phenyl)-4-dimethylamino-butyronitrile

-
-
74-86-2
acetylene

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| (η5-cyclopentadienyl)-η4-cycloocta-1,5-dienecobalt(I) In toluene at 140℃; under 10640 Torr; for 36h; | 75% |

| Conditions | Yield |
|---|---|
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; 0.8C12H6N2O4(2-)*HO(1-)*Al(3+)*0.4CF3O3S(1-)*0.1Ir(3+)*0.2C11H8N(1-)*0.1Cl(1-) In acetonitrile at 20℃; for 6h; Inert atmosphere; Irradiation; | 36% |

| Conditions | Yield |
|---|---|
| With sodium amide; benzene anschl. mit <2-Chlor-aethyl>-dimethyl-amin und anschl. Erhitzen mit wss. H2SO4; | |
| With sodium amide; benzene anschl. mit <2-Chlor-aethyl>-dimethyl-amin und anschl. Erhitzen mit NaOH in Butan-1-ol; |
-
-
4350-41-8
2-(4-chlorobenzyl)pyridine

-
-
107-99-3
2-(dimethylamino)ethyl chloride

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With potassium amide | |
| With sodium amide | |
| Stage #1: 2-(4-chlorobenzyl)pyridine With sodium amide In tetrahydrofuran at 20 - 25℃; for 1h; Stage #2: 2-(dimethylamino)ethyl chloride In tetrahydrofuran; toluene at 30 - 45℃; for 2h; |

| Conditions | Yield |
|---|---|
| With 2-pyridyllithium; diethyl ether |
-
-
88848-63-9
1t-(4-chloro-phenyl)-3-dimethylamino-1c-[2]pyridyl-propene

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; acetic acid Hydrogenation; |

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With sulfuric acid; water |

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With sulfuric acid; water |

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With formic acid; water; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With sodium amide; toluene anschl. mit wss. H2SO4; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: lithium diisopropylamide; HMPT / 2 h / 0 °C 1.2: 80 percent / HMPT / 3 h / -78 - 20 °C 2.1: 100 percent / 5 percent aq. HCl / methanol / 24 h / 20 °C 3.1: 100 percent / H2 / PtO2 / toluene / 12 h / 120 °C / 38000 Torr View Scheme |
-
-
160911-84-2
1-(4-chlorophenyl-1-(2-pyridyl))ehylene

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 30 percent / Na / 6 h / 20 °C 2: aq. potassium tert-butoxide / diethyl ether / 25 °C 3: 70 percent / tetrabutylammonium periodate / dioxane / 20 h / Heating 4: 100 percent / H2 / PtO2 / toluene / 12 h / 120 °C / 38000 Torr View Scheme |
-
-
233760-10-6
3-(p-chlorophenyl)-4,4-diethoxybutanenitrile

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 85 percent / (η5-cyclopentadienyl)<(1,5-η)-cyclooctadienyl>cobalt / toluene / 72 h / 120 °C / 9500 Torr 2: 100 percent / 5 percent aq. HCl / methanol / 24 h / 20 °C 3: 100 percent / H2 / PtO2 / toluene / 12 h / 120 °C / 38000 Torr View Scheme |

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: tetrabutylammonium hydrogen sulfate; carbon disulfide; 15 percent aq. NaOH / benzene / 0.5 h / 20 °C 2: 85 percent / (η5-cyclopentadienyl)<(1,5-η)-cyclooctadienyl>cobalt / toluene / 72 h / 120 °C / 9500 Torr 3: 100 percent / 5 percent aq. HCl / methanol / 24 h / 20 °C 4: 100 percent / H2 / PtO2 / toluene / 12 h / 120 °C / 38000 Torr View Scheme |
-
-
233760-12-8
2-[1-(4-chloro-phenyl)-2-[1,3]dioxolan-2-yl-ethyl]-pyridine

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent / 5 percent aq. HCl / methanol / 24 h / 20 °C 2: 100 percent / H2 / PtO2 / toluene / 12 h / 120 °C / 38000 Torr View Scheme |

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent / tetrabutylammonium periodate / dioxane / 20 h / Heating 2: 100 percent / H2 / PtO2 / toluene / 12 h / 120 °C / 38000 Torr View Scheme |
-
-
233760-14-0
N,N-dimethyl-4-(p-chlorophenyl)-4-(2-pyridyl)-2-hydroxybutanamide

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aq. potassium tert-butoxide / diethyl ether / 25 °C 2: 70 percent / tetrabutylammonium periodate / dioxane / 20 h / Heating 3: 100 percent / H2 / PtO2 / toluene / 12 h / 120 °C / 38000 Torr View Scheme |
-
-
42817-51-6
p-Chloro-N,N-dimethylcinnamylamine

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: H2 / HRh(CO)(PPh3)3 / benzene / 24 h / 80 °C / 76000 Torr 2: NH2OH 3: 92 percent / CS2, n-Bu4N(1+)*HSO4(1-), 15percent aq. NaOH / CH2Cl2 / 0.5 h / Ambient temperature 4: 75 percent / (η5-cyclopentadienyl)<(1,5-η)-cyclopentadienyl>cobalt / toluene / 36 h / 140 °C / 10640 Torr View Scheme |
-
-
158696-49-2
2-(4-Chloro-phenyl)-4-dimethylamino-butyraldehyde

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NH2OH 2: 92 percent / CS2, n-Bu4N(1+)*HSO4(1-), 15percent aq. NaOH / CH2Cl2 / 0.5 h / Ambient temperature 3: 75 percent / (η5-cyclopentadienyl)<(1,5-η)-cyclopentadienyl>cobalt / toluene / 36 h / 140 °C / 10640 Torr View Scheme |
-
-
158696-52-7
2-(4-Chloro-phenyl)-4-dimethylamino-butyraldehyde oxime

-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 92 percent / CS2, n-Bu4N(1+)*HSO4(1-), 15percent aq. NaOH / CH2Cl2 / 0.5 h / Ambient temperature 2: 75 percent / (η5-cyclopentadienyl)<(1,5-η)-cyclopentadienyl>cobalt / toluene / 36 h / 140 °C / 10640 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine hydrochloride; copper (II)-chloride 2: NaNH2 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine hydrochloride; copper (II)-chloride 2: NaNH2 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: cobaltocene / 4 h / 150 - 170 °C / Autoclave 2.1: sodium amide / tetrahydrofuran / 1 h / 20 - 25 °C 2.2: 2 h / 30 - 45 °C View Scheme |

| Conditions | Yield |
|---|---|
| With C17H24N5Ru(1+)*F6P(1-); potassium acetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 50℃; for 72h; Inert atmosphere; | 81% |
-
-
625-53-6
ethyl-2-thiourea

-
-
132-22-9
Chlorpheniramine

-
-
1378243-75-4
3-(4-ethylthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 78% |
-
-
598-52-7
1-(methyl)-thiourea

-
-
132-22-9
Chlorpheniramine

-
-
1378243-73-2
3-(4-methylthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 75% |
-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane at -10℃; for 2h; | 72.37% |
-
-
17356-08-0
thiourea

-
-
132-22-9
Chlorpheniramine

-
-
1377320-76-7
3-(4-thiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 67.7% |
-
-
132-22-9
Chlorpheniramine

-
-
103-85-5
monophenylthiourea

-
-
1378243-72-1
3-(4-phenylthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 67% |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-cyanoacetate; Chlorpheniramine With N-(2-methylnaphthalen-1-yl)-N’-(pyridin-2-ylmethyl)oxalamide; copper(I) bromide; sodium t-butanolate In isopropyl alcohol at 105℃; for 24h; Schlenk technique; Inert atmosphere; Stage #2: With water In isopropyl alcohol at 105℃; for 12h; Schlenk technique; Inert atmosphere; Cooling; | 66% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 65% |

| Conditions | Yield |
|---|---|
| With Cy-DHTP*HBF4; palladium diacetate; lithium tert-butoxide In toluene at 20 - 110℃; for 49h; Inert atmosphere; Sealed tube; | 53% |
-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| With deuterosulfonic acid In water-d2 at 120℃; for 6h; | 50% |

| Conditions | Yield |
|---|---|
| Stage #1: trifluoromethylsulfonic anhydride; Chlorpheniramine at -78℃; Inert atmosphere; Stage #2: triphenylphosphine Inert atmosphere; Cooling; Stage #3: Alkaline conditions; Inert atmosphere; regioselective reaction; | 40% |
| at -78 - 20℃; Alkaline conditions; | 40% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen; acetic acid In dimethyl sulfoxide at 120℃; for 16h; | A 13% B 26% |
-
-
132-22-9
Chlorpheniramine

-
-
21085-72-3
1-bromo-2,3,4-tri-O-acetyl-α-D-glucuronic acid methyl ester

-
-
145823-27-4
((2R,3R,4S,5S,6S)-6-Carboxy-3,4,5-trihydroxy-tetrahydro-pyran-2-yl)-[3-(4-chloro-phenyl)-3-pyridin-2-yl-propyl]-dimethyl-ammonium; chloride

| Conditions | Yield |
|---|---|
| With XAD-2 ion-exchange resin; sodium hydrogencarbonate In chloroform; water for 96h; Ambient temperature; | 15% |
-
-
132-22-9
Chlorpheniramine

-
-
32188-09-3
L-chlorpheniramine

| Conditions | Yield |
|---|---|
| With C18H18O6 |
-
-
132-22-9
Chlorpheniramine

| Conditions | Yield |
|---|---|
| Stage #1: Chlorpheniramine With sec.-butyllithium; (-)-sparteine In diethyl ether at -78℃; for 2h; Stage #2: With deuteromethanol at -78℃; |
Chlorpheniramine Chemical Properties
Chemistry informtion about Chlorpheniramine (CAS NO.132-22-9) is:
IUPAC Name: 3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine
Synonyms: Chlorpheniramine ; Dl-Chlorpheniramine
MF: C16H19ClN2
MW: 274.79
Density: 1.107 g/cm3
Flash Point: 183 °C
Boiling Point: 379 °C at 760 mmHg
Enthalpy of Vaporization: 62.7 kJ/mol
Vapour Pressure: 6.04E-06 mmHg at 25°C
Following is the molecular structure of Chlorpheniramine (CAS NO.132-22-9) is:
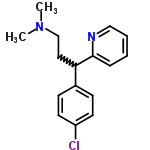
Chlorpheniramine Uses
Chlorpheniramine (CAS NO.132-22-9) is often combined with phenylpropanolamine to form an allergy medication with both antihistamine and decongestant properties.
Chlorpheniramine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 125mg/kg (125mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 92, Pg. 1339, 1972. | |
| mouse | LD50 | intravenous | 20mg/kg (20mg/kg) | Medizinische Welt. Vol. 17, Pg. 2791, 1966. | |
| mouse | LD50 | oral | 121mg/kg (121mg/kg) | Medizinische Welt. Vol. 17, Pg. 2791, 1966. | |
| mouse | LD50 | subcutaneous | 160mg/kg (160mg/kg) | Bollettino Chimico Farmaceutico. Vol. 111, Pg. 293, 1972. | |
| rabbit | LD50 | intravenous | 22mg/kg (22mg/kg) | Medizinische Welt. Vol. 17, Pg. 2791, 1966. | |
| rat | LD50 | oral | 118mg/kg (118mg/kg) | Medizinische Welt. Vol. 17, Pg. 2791, 1966. |
Chlorpheniramine Consensus Reports
Reported in EPA TSCA Inventory.
Chlorpheniramine Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. When heated to decomposition it emits toxic vapors of NOx and Cl−.
Related Products
- Chlorpheniramine
- Chlorpheniramine maleate
- 13223-25-1
- 13223-43-3
- 132234-68-5
- 132234-69-6
- 132236-08-9
- 132237-63-9
- 1322-40-3
- 132244-31-6
- 132245-57-9
- 13224-84-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View