-
Name
Chlorpromazine
- EINECS 200-045-8
- CAS No. 50-53-3
- Article Data36
- CAS DataBase
- Density 1.212g/cm3
-
Solubility
Soluble
- Hazard Symbols 6.1 (Packing Group: III) UN NO.
- Synonyms Phenothiazine,2-chloro-10-[3-(dimethylamino)propyl]- (7CI,8CI);2-Chloro-10-[3-(dimethylamino)propyl]phenothiazine; 2-Chloropromazine; 2601A;Aminazin; Aminazine; Ampliactil; Amplictil; BC 135; CPZ; Chlor-Promanyl;Chlordelazin; Chlorderazin; Chlorpromados; Chlorpromazine; Chlropromados;Contomin; Elmarin; Esmind; Fenactil; Fenaktyl; Fraction AB; HL 5746;Largactilothiazine; Largactyl; Megaphen; NSC 167745; NSC 226514; Noiafren;Novomazina; Phenactyl; Proma; Promactil; Promazil; Propaphenin; Prozil; RP4560; SKF 2601A; Sanopron; Sedatil; Thorazin; Thorazine; Wintermin
- PSA 31.78000
- LogP 4.95940
Synthetic route
-
-
1219602-20-6
C11H16ClIN2

-
-
6320-02-1
2-bromothiophenol

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; L-proline In ethyl methyl ether at 90 - 110℃; Inert atmosphere; | 78% |
-
-
50-00-0
formaldehyd

-
-
2095-17-2
2-chloro-10-(3-aminopropyl)phenothiazine

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With formic acid In water at 80℃; for 8h; Eschweiler-Clark Amine Methylation; Microwave irradiation; | 66% |
-
-
31928-44-6
2-bromo-4-chloroiodobenzene

-
-
6320-02-1
2-bromothiophenol

-
-
109-55-7
1-amino-3-(dimethylamino)propane

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate In toluene at 60 - 160℃; for 2.33333h; Microwave irradiation; | 50% |

-
-
92-39-7
2-chlorophenothiazine

-
-
109-54-6
3-(Dimethylamino)propyl chloride

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With sodium amide | |
| (i) NaNH2, toluene, (ii) /BRN= 605294/; Multistep reaction; | |
| With potassium hydroxide; potassium carbonate; tetra(n-butyl)ammonium hydrogensulfate toluene 1.) room temp., 2 h, 2.) reflux, 18 h; Yield given. Multistep reaction; |
-
-
3179-63-3
3-Dimethylamino-1-propanol

-
-
36798-98-8
2-chloro-phenothiazine-10-carbonyl chloride

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With toluene Erhitzen des Reaktionsprodukts unter vermindertem Druck auf 210-220grad; |
-
-
4414-83-9
3-(2-chloro-10H-phenothiazin-10-yl)propanonitrile

-
A
-
2095-17-2
2-chloro-10-(3-aminopropyl)phenothiazine

-
B
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With nickel; dimethyl amine; benzene at 110 - 116℃; under 58840.6 Torr; Hydrogenation; |
-
-
109454-48-0
N'-[2-(2-bromo-phenylsulfanyl)-5-chloro-phenyl]-N,N-dimethyl-propanediyldiamine

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With iron; acetic acid; xylene |
-
-
41951-57-9
1-(2-chloro-phenothiazin-10-yl)-3-methanesulfonyloxy-propane

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With dimethyl amine |
-
-
103278-35-9
bis-[3-(2-chloro-phenothiazin-10-yl)-propyl]-methyl-amine

-
A
-
63615-79-2
10-Allyl-2-chlorophenothiazine

-
B
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; methyl iodide |
-
-
2765-59-5
2-chloro-10-(3-chloropropyl)-10H-phenothiazine

-
-
124-40-3
dimethyl amine

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| In ethanol |
-
-
51-61-6
dopamine

-
-
50-53-3
chloropromazine cation radical

-
A
-
50673-96-6
dopaminoquinone

-
B
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| In hydrogenchloride Rate constant; pH=1.5; |

-
-
92-39-7
2-chlorophenothiazine

-
-
109-54-6
3-(Dimethylamino)propyl chloride

-
A
-
19607-03-5
2-chloro-10-methyl-10H-phenothiazine

-
B
-
63615-79-2
10-Allyl-2-chlorophenothiazine

-
C
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chlorobenzene; toluene for 4.5h; Heating; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride 1.) THF, -45 deg C - 60 deg C, 2.) 60 deg C, 10 min; Yield given. Multistep reaction; |
-
-
138-65-8
Norepinephrine

-
-
50-53-3
chloropromazine cation radical

-
A
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
B
-
14309-96-7
4-(2-Amino-1-hydroxy-ethyl)-[1,2]benzoquinone

| Conditions | Yield |
|---|---|
| In hydrogenchloride Rate constant; pH=7; |

-
-
329-65-7
Epinephrine

-
-
50-53-3
chloropromazine cation radical

-
A
-
672-73-1, 162706-73-2
adrenaline o-quinone

-
B
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| In hydrogenchloride Rate constant; pH=7; |

-
-
58-15-1
aminopyrine

-
A
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
B
-
58-15-1
aminopyrine radical cation

| Conditions | Yield |
|---|---|
| Rate constant; pH 6; |

-
-
76637-15-5
[3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-(2-methyl-acryloyloxymethyl)-ammonium; chloride

-
A
-
50-00-0
formaldehyd

-
B
-
79-41-4
poly(methacrylic acid)

-
C
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| In water at 21℃; pH=8.8, ionic strength 0.1 M KCl; other pH, other temp.; hydrolysis rate; |

-
-
76637-14-4
Benzoyloxymethyl-[3-(2-chloro-phenothiazin-10-yl)-propyl]-dimethyl-ammonium; chloride

-
A
-
50-00-0
formaldehyd

-
B
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
C
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| In water at 21℃; pH=8.2, ionic strength 0.1 M KCl; other pH; hydrolysis rate; |

-
-
51-61-6
dopamine

-
A
-
50673-96-6
dopaminoquinone

-
B
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| Kinetics; Rate constant; in constant ionic strength (0.8 M) mixtures of HClO4 and NaClO4 in 40percent MeO/H2O (w/w) with a pH between 0.1 and 1.5; |

-
-
50-53-3
chloropromazine cation radical

-
-
452-86-8
4-methyl-1,2-dihydroxybenzene

-
A
-
3131-54-2
4-methylbenzo-1,2-quinone

-
B
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| In hydrogenchloride Rate constant; pH=7; |


-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; acetic acid Hydrogenation; |

| Conditions | Yield |
|---|---|
| With n-butyllithium; diethyl ether |

| Conditions | Yield |
|---|---|
| With xylene |
-
-
3953-65-9
2-chloro-N-methyl-10H-phenothiazine-10-propanamine hydrochloride

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (NH4)2SO4 / 110 °C 2: 2.) LiAlH4 / 1.) THF, -45 deg C - 60 deg C, 2.) 60 deg C, 10 min View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous H2O2; ethanol 2: iron-powder; acetic acid; xylene View Scheme | |
| With potassium carbonate In water pH=10; Inert atmosphere; |
-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| In acetone at 40℃; for 48h; Inert atmosphere; Reflux; | 97% |

| Conditions | Yield |
|---|---|
| With methanesulfonato(2-dicyclohexylphosphino-3,6-dimethoxy-2',4',6'-tri-i-propyl-1,1'-biphenyl)(2'-methylamino-1,1'-biphenyl-2-yl)palladium(II); potassium hydroxide In water; toluene at 110℃; Flow reactor; | 95% |
-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| With GLUTATHIONE; isopropyl alcohol; acetone In water Rate constant; Product distribution; Irradiation; pH=3; pulse radiolysis; other thiol reagents and CPZ concentrations; | 91% |
| With halothane peroxyl radical In various solvent(s) Rate constant; Ambient temperature; Irradiation; pH=7; pulse radiolysis; different CZ concentrations; | 54 % Spectr. |
| With dihydrogen peroxide; horseradish peroxidase In acetate buffer pH=4.5; Kinetics; Further Variations:; Reagents; Oxidation; | |
| With hydroxyl pH=7; Kinetics; aq. phosphate buffer; |
-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
3926-64-5
10-[3-(dimethylamino)propyl]-10H-phenothiazin-2-ol

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine; boric acid; palladium diacetate; caesium carbonate In 1-methyl-pyrrolidin-2-one at 100℃; for 14h; Schlenk technique; Inert atmosphere; | 87% |
| With water for 120h; Irradiation; |

| Conditions | Yield |
|---|---|
| In acetonitrile Electrolysis; | 83% |
| With hydrogenchloride; sodium nitrite In water for 2h; Product distribution; oxidation of phenothiazine salts to their sulfoxides with aqueous nitrous acid; hydrogen peroxide oxidation; | 74% |
| With hydrogenchloride; sodium nitrite In water for 2h; | 74% |
-
-
5335-05-7
chloromethyl benzoate

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
76637-14-4
Benzoyloxymethyl-[3-(2-chloro-phenothiazin-10-yl)-propyl]-dimethyl-ammonium; chloride

| Conditions | Yield |
|---|---|
| In acetone | 81.5% |
-
-
50893-53-3
carbonochloridic acid 1-chloro-ethyl ester

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane for 1h; Substitution; Heating; | 81% |
-
-
27550-73-8
Chloromethyl methacrylate

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
76637-15-5
[3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-(2-methyl-acryloyloxymethyl)-ammonium; chloride

| Conditions | Yield |
|---|---|
| In acetone | 79% |

| Conditions | Yield |
|---|---|
| With Cy-DHTP*HBF4; palladium diacetate; lithium tert-butoxide In toluene at 20 - 110℃; for 21h; Inert atmosphere; Sealed tube; | 65% |
-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
21085-72-3
1-bromo-2,3,4-tri-O-acetyl-α-D-glucuronic acid methyl ester

-
-
86492-49-1
chlorpromazine N+-glucuronide chloride

| Conditions | Yield |
|---|---|
| With XAD-2 ion-exchange resin; sodium hydrogencarbonate In water; benzene for 72h; Ambient temperature; | 46% |
-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
21085-72-3
1-bromo-2,3,4-tri-O-acetyl-α-D-glucuronic acid methyl ester

-
A
-
86492-49-1
chlorpromazine N+-glucuronide chloride

-
B
-
145823-11-6
[3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-((2R,3R,4S,5S,6S)-3,4,5-triacetoxy-6-methoxycarbonyl-tetrahydro-pyran-2-yl)-ammonium; bromide

-
C
-
145823-13-8
Hydrogen carbonate[3-(2-chloro-phenothiazin-10-yl)-propyl]-dimethyl-((2R,3R,4S,5S,6S)-3,4,5-triacetoxy-6-carboxy-tetrahydro-pyran-2-yl)-ammonium;

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; benzene for 72h; Ambient temperature; | A 46% B n/a C n/a |

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
A
-
1672-76-0
Chlorpromazine oxide

-
B
-
969-99-3
chlorpromazine sulfoxide

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid; sodium hydroxide In dichloromethane at 0℃; for 4h; | A 40% B 10% |
-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
A
-
2095-62-7
7-hydroxychlorpromazine

-
B
-
3926-66-7
2-chloro-10-(3-dimethylamino-propyl)-10H-phenothiazin-1-ol

-
C
-
3930-47-0
3-hydroxyl CPZ

-
D
-
969-99-3
chlorpromazine sulfoxide

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine With ethylenediaminetetraacetic acid trisodium salt; oxygen; manganese (II) acetate tetrahydrate; ascorbic acid In water pH=4; Udenfriend reaction; Stage #2: With ferrous(II) sulfate heptahydrate; ethylenediaminetetraacetic acid trisodium salt; oxygen In water at 45℃; for 2h; Udenfriend reaction; | A n/a B 0.4% C 0.84% D 0.58% |

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
10404-90-7
chlorpromazine N',S-dioxide

| Conditions | Yield |
|---|---|
| With ethanol; dihydrogen peroxide | |
| With dihydrogen peroxide In ethanol | |
| With dihydrogen peroxide In ethanol for 24h; Ambient temperature; | |
| Multi-step reaction with 2 steps 1: aq. H2O2, NH3 / ethanol / 60 h 2: aq. H2O2 / ethanol / 84 h View Scheme | |
| Multi-step reaction with 2 steps 1: 3-chloro-benzenecarboperoxoic acid; sodium hydroxide / dichloromethane / 4 h / 0 °C 2: 3-chloro-benzenecarboperoxoic acid; sodium hydroxide / dichloromethane / 4 h / 0 °C View Scheme |
-
-
1556-34-9
9-(bromomethyl)acridine

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
56265-45-3
acridin-9-ylmethyl-[3-(2-chloro-phenothiazin-10-yl)-propyl]-dimethyl-ammonium; bromide

| Conditions | Yield |
|---|---|
| In acetonitrile Ambient temperature; |
-
-
14181-72-7, 17082-13-2, 17082-14-3
2-bromo-1-phenyl-ethanone oxime

-
-
50-53-3
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

-
-
19337-44-1
[3-(2-chloro-phenothiazin-10-yl)-propyl]-(2-hydroxyimino-2-phenyl-ethyl)-dimethyl-ammonium; bromide

| Conditions | Yield |
|---|---|
| In diethyl ether |
Chlorpromazine Chemical Properties
MF: C17H19ClN2S
MW: 318.86416
MS: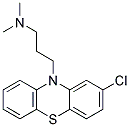
EINECS: 200-045-8
Product Categories:
Mol File: 50-53-3.mol
Surface Tension: 46.6 dyne/cm
Density: 1.212 g/cm3
Flash Point: 226 °C
Enthalpy of Vaporization: 70.89 kJ/mol
Boiling Point: 450.1 °C at 760 mmHg
Vapour Pressure: 2.72E-08 mmHg at 25°C
Chlorpromazine Uses
3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine(50-53-3) have good effect on have good effect on schizophrenia, severe depression and anxiety, hyperactive children, you can also use 3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine(50-53-3) to relieve nausea, belching.
Chlorpromazine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD50 | subcutaneous | > 10mg/kg (10mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. | |
| cat | LDLo | oral | 100mg/kg (100mg/kg) | behavioral: altered sleep time (including change in righting reflex) behavioral: regidity gastrointestinal: nausea or vomiting | Oyo Yakuri. Pharmacometrics. Vol. 11, Pg. 561, 1976. |
| child | LDLo | oral | 37500ug/kg (37.5mg/kg) | behavioral: convulsions or effect on seizure threshold behavioral: coma | New England Journal of Medicine. Vol. 256, Pg. 527, 1957. |
| dog | LD50 | intravenous | 30mg/kg (30mg/kg) | ungs, thorax, or respiration: emphysema blood: hemorrhage lungs, thorax, or respiration: acute pulmona | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 118, Pg. 358, 1959. |
| dog | LD50 | subcutaneous | > 20mg/kg (20mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. | |
| dog | LDLo | oral | 250mg/kg (250mg/kg) | behavioral: altered sleep time (including change in righting reflex) behavioral: regidity gastrointestinal: nausea or vomiting | Oyo Yakuri. Pharmacometrics. Vol. 11, Pg. 561, 1976. |
| guinea pig | LD50 | intraperitoneal | 87mg/kg (87mg/kg) | Medicina Experimentalis. Vol. 8, Pg. 237, 1963. | |
| guinea pig | LDLo | oral | 250mg/kg (250mg/kg) | behavioral: altered sleep time (including change in righting reflex) behavioral: regidity gastrointestinal: nausea or vomiting | Oyo Yakuri. Pharmacometrics. Vol. 11, Pg. 561, 1976. |
| human | TDLo | oral | 8570ug/kg/12D (8.57mg/kg) | behavioral: wakefulness behavioral: muscle weakness behavioral: tremor | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 93, 1972. |
| infant | TDLo | oral | 20mg/kg (20mg/kg) | vascular: bp lowering not characterized in autonomic section behavioral: somnolence (general depressed activity) | American Journal of Diseases of Children. Vol. 130, Pg. 507, 1976. |
| mammal (species unspecified) | LD50 | oral | 500mg/kg (500mg/kg) | behavioral: anticonvulsant behavioral: alteration of classical conditioning | Journal of Medicinal Chemistry. Vol. 8, Pg. 836, 1965. |
| man | TDLo | oral | 8900ug/kg (8.9mg/kg) | lungs, thorax, or respiration: dyspnea | American Journal of Emergency Medicine. Vol. 14, Pg. 467, 1996. |
| man | TDLo | oral | 217mg/kg/19D (217mg/kg) | blood: agranulocytosis skin and appendages (skin): "dermatitis, allergic: after systemic exposure" | Journal of Clinical Psychiatry. Vol. 46, Pg. 341, 1985. |
| monkey | LD50 | subcutaneous | > 5mg/kg (5mg/kg) | blood: agranulocytosis
| Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. |
| mouse | LC50 | inhalation | 209mg/m3/2H (209mg/m3) | behavioral: muscle weakness cardiac: pulse rate increase without fall in bp blood: leukopenia | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 10, Pg. 73, 1968. |
| mouse | LD50 | intraperitoneal | 14mg/kg (14mg/kg) | Farmaco, Edizione Scientifica. Vol. 14, Pg. 269, 1959. | |
| mouse | LD50 | intravenous | 16mg/kg (16mg/kg) | behavioral: muscle weakness cardiac: pulse rate increase without fall in bp blood: leukopenia | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. |
| mouse | LD50 | oral | 135mg/kg (135mg/kg) | lbehavioral: convulsions or effect on seizure threshold lungs, thorax, or respiration: other changes | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 118, Pg. 358, 1959. |
| mouse | LD50 | subcutaneous | 33mg/kg (33mg/kg) | behavioral: ataxia behavioral: regidity | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. |
| mouse | LD50 | unreported | 82500ug/kg (82.5mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 53, Pg. 2S, 1957. | |
| mouse | LDLo | intramuscular | 300mg/kg (300mg/kg) | Therapie. Vol. 15, Pg. 1064, 1960. | |
| rabbit | LD50 | intravenous | 16mg/kg (16mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 120, Pg. 450, 1959. | |
| rat | LC50 | inhalation | 209mg/m3/2H (209mg/m3) | behavioral: muscle weakness cardiac: pulse rate increase without fall in bp blood: leukopenia | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 10, Pg. 73, 1968. |
| rat | LD50 | intraperitoneal | 137mg/kg (137mg/kg) | German Offenlegungsschrift Patent Document. Vol. #2350222, | |
| rat | LD50 | intravenous | 23mg/kg (23mg/kg) | behavioral: muscle weakness cardiac: pulse rate increase without fall in bp blood: leukopenia | Farmaco, Edizione Pratica. Vol. 26, Pg. 585, 1971. |
| rat | LD50 | oral | 142mg/kg (142mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 18, Pg. 261, 1968. | |
| rat | LD50 | subcutaneous | 75mg/kg (75mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 56, Pg. 377, 1960. | |
| women | TDLo | oral | 200ug/kg (0.2mg/kg) | behavioral: sleep liver: "jaundice, other or unclassified" | New York State Journal of Medicine. Vol. 57, Pg. 1922, 1957. |
| women | TDLo | oral | 16800ug/kg/3W (16.8mg/kg) | blood: agranulocytosis | Postgraduate Medical Journal. Vol. 69, Pg. 885, 1993. |
| women | TDLo | oral | 166mg/kg/18D- (166mg/kg) | blood: other hemolysis with or without anemia blood: other changes | Postgraduate Medical Journal. Vol. 53, Pg. 278, 1977. |
| women | TDLo | oral | 240mg/kg/30D- (240mg/kg) | blood: agranulocytosis blood: changes in leucocyte (wbc) count blood: agranulocytosis | Journal of Clinical Psychiatry. Vol. 46, Pg. 341, 1985. |
| women | TDLo | oral | 240mg/kg/30D (240mg/kg) | skin and appendages (skin): "dermatitis, allergic: after systemic exposure" | Journal of Clinical Psychiatry. Vol. 46, Pg. 341, 1985. |
Chlorpromazine Specification
IUPAC: 3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine(50-53-3)
CAS: 50-53-3
Synonyms: 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-;2601-A;2-Chloro-10-[3-(dimethyamino)propyl]phenothiazine;2-Chloropromazine;2-Cloro-10 (3-dimetilaminopropil)fenotiazina;3-(2-Chloro-10H-phenothiazin-10-yl)-N,N-dimethyl-1-propanamine;4560 R.P.;Aminasine
Related Products
- Chlorpromazine
- Chlorpromazine hydrochloride
- 50533-97-6
- 5053-43-0
- 50534-45-7
- 50534-49-1
- 50536-49-7
- 50541-93-0
- 50543-23-2
- 50543-78-7
- 505-44-2
- 50544-72-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View