-
Name
D-alpha-Tocopheryl acetate
- EINECS 200-405-4
- CAS No. 58-95-7
- Article Data58
- CAS DataBase
- Density 0.941 g/cm3
- Solubility <0.1 g/100 mL at 17 °C in water
- Melting Point ~25 °C(lit.)
- Formula C31H52O3
- Boiling Point 485.3 °C at 760 mmHg
- Molecular Weight 472.752
- Flash Point 235.6 °C
- Transport Information
- Appearance off-white crystalline solid
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms E-Vicotrat;Endo E Dompe;Ephynal acetate;Epsilan-M;Erevit;Nanotopes;Natur-E granulate;Tofaxin;Tokoferol acetate;2H-1-Benzopyran-6-ol,3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-, acetate,[2R-[2R*(4R*,8R*)]]-;2H-1-Benzopyran-6-ol,3,4-dihydro-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-, acetate,(2R)- (9CI);Vitamin E acetate (7CI);a-Tocopherol acetate (6CI);(+)-a-Tocopherol acetate;(+)-a-Tocopheryl acetate;(2R,4'R,8'R)-a-Tocopherolacetate;(2R,4'R,8'R)-a-Tocopheryl acetate;(R,R,R)-a-Tocopheryl acetate;Alfacol;Combinal E;Contopheron;Copherol F 1250C;Covitol;
- PSA 35.53000
- LogP 9.05990
Synthetic route

| Conditions | Yield |
|---|---|
| With dmap In toluene at 65℃; for 5h; Solvent; Large scale; | 99.37% |
| indium(III) triflate at 25 - 32℃; for 4.66667h; Product distribution / selectivity; | 97% |
| 11percent Ca +50 percent Na on SiO2 prepared from Ca(OH)2 + Na silicate at 100℃; for 3.5h; | 97.7% |

| Conditions | Yield |
|---|---|
| 92% |

| Conditions | Yield |
|---|---|
| With 1H,4H-piperazine-N,N'-diium hydrogensulfate at 80℃; for 4h; | 89.2% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sulfuric acid; oxalic acid; acetic anhydride In tetralin; n-heptane | 89% |
-
-
186537-57-5
dl-α-tocopheryl acetate

-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| durch fraktionierte Krystallisation; |
-
-
108-24-7
acetic anhydride

-
-
59-02-9
Tocopherol

-
A
-
64-19-7
acetic acid

-
B
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| With sulfuric acid at 60℃; Kinetics; Equilibrium constant; Further Variations:; Temperatures; Acetylation; |

-
-
59965-06-9
Vitamin E benzyl ether

-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / Pd/C 2: pyridine View Scheme |
-
-
59965-21-8
(S)-6-benzyloxy-2,5,7,8-tetramethylchroman-2-ethanol p-toluenesulfonate

-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 93 percent / Li2CuCl4 2: H2 / Pd/C 3: pyridine View Scheme |

-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 93 percent / Li2CuCl4 2: H2 / Pd/C 3: pyridine View Scheme |
-
-
219718-22-6
4-acetoxy-2,3,5-trimethyl-6-butoxymethyl-1-hydroxybenzene

-
-
2140-82-1, 60976-73-0
2,6,10,14-tetramethyl-1-pentadecene

-
A
-
36592-62-8
4-acetoxy-2,3,5-trimethylphenol

-
B
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| In butan-1-ol |


-
-
1169860-40-5
C39H55ClO5

-
-
108-24-7
acetic anhydride

-
A
-
54165-54-7
2S,4'R,8'R-α-tocopheryl acetate

-
B
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Stage #1: C39H55ClO5 With lithium aluminium tetrahydride In tetrahydrofuran for 18h; Reflux; Stage #2: acetic anhydride In pyridine at 25℃; for 4h; Stage #3: With chiral Iridium catalyst; hydrogen In dichloromethane at 25℃; under 37503.8 Torr; for 3h; |

-
-
403815-06-5
(R)-2,5,7,8-tetramethyl-2-((3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl)chroman-6-yl acetate

-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| With C30H42IrNOP(1+)*C32H12BF24(1-); hydrogen In dichloromethane at 20℃; under 37503.8 Torr; for 2h; optical yield given as %de; stereoselective reaction; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic anhydride; acetic acid at 70℃; for 1h; Reagent/catalyst; Solvent; Temperature; Industrial scale; |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
113892-10-7
(2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-2H-chromen-6-yl acetate

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 24h; Heating; | 93% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 4h; Heating / reflux; | 88% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 24h; Heating / reflux; | 88% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene Oxidation; Heating; | 72% |
-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| With nitric acid; acetic acid Ambient temperature; | 75% |
| With nitric acid; acetic acid; sodium nitrite at 20℃; for 0.5h; | 74% |

| Conditions | Yield |
|---|---|
| With tetrakis[(R)-(1-adamantyl)-(N-phthalimido)acetate]dirhodium(II) In dichloromethane at 24℃; diastereoselective reaction; | 60% |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
7559-04-8
2-[(3R,7R,11R)-3-hydroxy-3,7,11,15-tetramethylhexadecyl]-3,5,6-trimethyl-2,5-cyclohexadiene-1,4-dione

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII) In ethanol; water at 25℃; for 10h; | 42% |
| Multi-step reaction with 2 steps 1: potassium hydroxide / ethanol / 1 h / 10 °C 2: iron(III) chloride; water View Scheme |

| Conditions | Yield |
|---|---|
| With dimyristoylphosphatidylcholine; Tris buffer; sodium cholate Rate constant; pure porcine cholesterol esterase (PCE); other bile salts, other carboxylic ester hydrolase; |

| Conditions | Yield |
|---|---|
| With cholesterol esterase; tris-buffer; chenodeoxycholate; dimyristoylphosphatidylcholine at 37℃; initial rates of the reaction; other dihydroxy and trihydroxy bile salts; also epimeric tocopheryl acetate; | |
| With dimyristoylphosphatidylcholine; bovine cholesterol esterase; Tris buffer; sodium chloride at 37℃; also racemic and epimeric tocopherol; noncompetitive and competitive hydrolysis; other enzyme; | |
| Stage #1: RRR-α-tocopheryl acetate With ethanol; potassium hydroxide at 10 - 12℃; for 1h; Industry scale; Stage #2: With hydrogenchloride In ethanol; water |
-
-
64-19-7
acetic acid

-
-
58-95-7
RRR-α-tocopheryl acetate

-
A
-
108-24-7
acetic anhydride

-
B
-
59-02-9
Tocopherol

| Conditions | Yield |
|---|---|
| With sulfuric acid at 60℃; Equilibrium constant; Further Variations:; Temperatures; Hydrolysis; |

-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
131615-85-5
2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-3,4-dihydro-2H-chromene-5,6-dione

| Conditions | Yield |
|---|---|
| With nitric acid; acetic acid In dichloromethane at 20℃; for 0.5h; |
-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane |
-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 93 percent / DDQ / toluene / 24 h / Heating 2: 74 percent / NBS / 1,2-dimethoxy-ethane; H2O / 24 h / 4 °C 3: 8 percent / ZnO / ethanol / 4 h / 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
428505-71-9
6-hydroxy-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-4-chromanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 93 percent / DDQ / toluene / 24 h / Heating 2: 74 percent / NBS / 1,2-dimethoxy-ethane; H2O / 24 h / 4 °C 3: 75 percent / ZnO / ethanol / 4 h / 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 93 percent / DDQ / toluene / 24 h / Heating 2: 74 percent / NBS / 1,2-dimethoxy-ethane; H2O / 24 h / 4 °C 3: 75 percent / ZnO / ethanol / 4 h / 20 °C 4: 6 percent / sodium dodecyl sulfate; aq. H2O2 / aq. phosphate buffer / 72 h / 20 °C / pH 6 View Scheme | |
| Multi-step reaction with 5 steps 1: 93 percent / DDQ / toluene / 24 h / Heating 2: 74 percent / NBS / 1,2-dimethoxy-ethane; H2O / 24 h / 4 °C 3: 75 percent / ZnO / ethanol / 4 h / 20 °C 4: 11 percent / sodium dodecyl sulfate / aq. phosphate buffer / 72 h / 20 °C / pH 6 5: H2O2 / 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 93 percent / DDQ / toluene / 24 h / Heating 2: 74 percent / NBS / 1,2-dimethoxy-ethane; H2O / 24 h / 4 °C 3: 75 percent / ZnO / ethanol / 4 h / 20 °C 4: 11 percent / sodium dodecyl sulfate / aq. phosphate buffer / 72 h / 20 °C / pH 6 View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
428505-70-8
6-acetoxy-3-bromo-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-4-chromanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93 percent / DDQ / toluene / 24 h / Heating 2: 74 percent / NBS / 1,2-dimethoxy-ethane; H2O / 24 h / 4 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
113892-09-4
(2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyl-tridecyl]-2H-chromen-6-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 72 percent / DDQ / toluene / Heating 2: 76 percent / K2CO3 / methanol / 8 h / 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 72 percent / DDQ / toluene / Heating 2.1: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3.1: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4.1: 85 percent / Jacobsen's (S,S)-Salen reagent; 4-(3-phenylpropyl)pyridine N-oxide; aq. NaOCl / CH2Cl2 / 2 h / 0 °C 5.1: 24.1 mg / LiAlH4; AlCl3 / tetrahydrofuran / 3 h / 0 °C 6.1: dimethylaniline / CH2Cl2 / 0.25 h 6.2: Et3N / CH2Cl2 / 0 - 20 °C 7.1: TBAF / tetrahydrofuran / 0 - 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: 72 percent / DDQ / toluene / Heating 2.1: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3.1: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4.1: 44.3 percent / H2O; NBS / 1,2-dimethoxy-ethane / 0 - 4 °C 5.1: NaH / tetrahydrofuran / 6 h / 20 °C 6.1: 24.1 mg / LiAlH4; AlCl3 / tetrahydrofuran / 3 h / 0 °C 7.1: dimethylaniline / CH2Cl2 / 0.25 h 7.2: Et3N / CH2Cl2 / 0 - 20 °C 8.1: TBAF / tetrahydrofuran / 0 - 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 72 percent / DDQ / toluene / Heating 2.1: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3.1: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4.1: 85 percent / Jacobsen's (R,R)-Salen reagent; 4-(3-phenylpropyl)pyridine N-oxide; aq. NaOCl / CH2Cl2 / 2 h / 0 °C 5.1: 25.2 mg / LiAlH4; AlCl3 / tetrahydrofuran / 3 h / 0 °C 6.1: dimethylaniline / CH2Cl2 / 0.25 h 6.2: 82.7 percent / Et3N / CH2Cl2 / 0 - 20 °C 7.1: 85 percent / TBAF / tetrahydrofuran / 0 - 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: 72 percent / DDQ / toluene / Heating 2.1: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3.1: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4.1: 51.8 percent / H2O; NBS / 1,2-dimethoxy-ethane / 0 - 4 °C 5.1: NaH / tetrahydrofuran / 6 h / 20 °C 6.1: 25.2 mg / LiAlH4; AlCl3 / tetrahydrofuran / 3 h / 0 °C 7.1: dimethylaniline / CH2Cl2 / 0.25 h 7.2: 82.7 percent / Et3N / CH2Cl2 / 0 - 20 °C 8.1: 85 percent / TBAF / tetrahydrofuran / 0 - 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
269726-01-4
6-{[tert-butyl(dimethyl)-silyl]-oxy}-(2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyl-tridecyl]-2H-chromene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 72 percent / DDQ / toluene / Heating 2: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
269725-99-7
(1aS,2R,7bS)-2,4,5,7-tetramethyl-2-[(4R,8R)-4,8,12-trimethyl-tridecyl]-1a,7b-dihydro-2H-oxireno[2,3-c]chromen-6-yl tert-butyl(dimethyl)silyl ether

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 72 percent / DDQ / toluene / Heating 2: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4: 85 percent / Jacobsen's (S,S)-Salen reagent; 4-(3-phenylpropyl)pyridine N-oxide; aq. NaOCl / CH2Cl2 / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 72 percent / DDQ / toluene / Heating 2: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4: 44.3 percent / H2O; NBS / 1,2-dimethoxy-ethane / 0 - 4 °C 5: NaH / tetrahydrofuran / 6 h / 20 °C View Scheme |
-
-
58-95-7
RRR-α-tocopheryl acetate

-
-
269725-89-5
(1aR,2R,7bR)-2,4,5,7-tetramethyl-2-[(4R,8R)-4,8,12-trimethyl-tridecyl]-1a,7b-dihydro-2H-oxireno[2,3-c]chromen-6-yl tert-butyl(dimethyl)silyl ether

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 72 percent / DDQ / toluene / Heating 2: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4: 85 percent / Jacobsen's (R,R)-Salen reagent; 4-(3-phenylpropyl)pyridine N-oxide; aq. NaOCl / CH2Cl2 / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 72 percent / DDQ / toluene / Heating 2: 76 percent / K2CO3 / methanol / 8 h / 20 °C 3: 98 percent / 2,6-lutidine / CH2Cl2 / 3.5 h / 20 °C 4: 51.8 percent / H2O; NBS / 1,2-dimethoxy-ethane / 0 - 4 °C 5: NaH / tetrahydrofuran / 6 h / 20 °C View Scheme |
D-alpha-Tocopheryl acetate Chemical Properties
D-α-Tocopheryl acetate(58-95-7) is also named as D-alpha-Tocopheryl acetate;(2r,4’r,8’r)-alpha-tocopherylacetate;(2R,4’R,8’R)-O-Acetyl-α-tocopherol;(r,r,r)-alpha-tocopherylacetate;[2R-[2R*(4R8,8R*)]]-3,4-Dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-ol acetate;2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-,acetate,(+)-6-chromano,and so on.D-α-Tocopheryl acetate(58-95-7) is odorless off-white crystals.
CAS: 58-95-7
Molecular Formula: C31H52O3
Molecular Weight: 472.74
Molecular structure: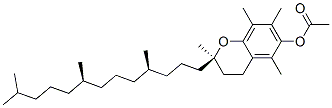
EINECS: 231-710-0
Melting point: ~25 °C(lit.)
alpha: 3 °(c=2, in ethanol 25 °C)
Boiling point: 224 °C0.3 mm Hg(lit.)
Density: 0.953 g/mL at 25 °C(lit.)
Refractive index: n20/D 1.496(lit.)
Flash point: >230 °F
Storage temp.: 2-8°C
Form: oil or semi-solid
Color: yellow
Water Solubility: <0.1 g/100 mL at 17 °C
Merck: 9495
D-alpha-Tocopheryl acetate Uses
D-alpha-Tocopheryl acetate Toxicity Data With Reference
D-alpha-Tocopheryl acetate Safety Profile
When heated to decomposition it emits acrid smoke and irritating fumes.
Safety Statements: 24/25
WGK Germany: 1
Related Products
- D-alpha-Tocopheryl acetate
- 589-57-1
- 58957-92-9
- 58958-60-4
- 589-59-3
- 58960-71-7
- 5896-17-3
- 58962-34-8
- 589-65-1
- 58965-66-5
- 589-66-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View