-
Name
DIETHYL BROMOMALONATE
- EINECS 211-683-1
- CAS No. 685-87-0
- Article Data49
- CAS DataBase
- Density 1.452 g/cm3
- Solubility Does not mix well with water.
- Melting Point -54°C
- Formula C7H11BrO4
- Boiling Point 233.999 °C at 760 mmHg
- Molecular Weight 239.066
- Flash Point 89.603 °C
- Transport Information UN 3265 8/PG 2
- Appearance Clear colourless to slightly yellow liquid
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Malonicacid, bromo-, diethyl ester (6CI,7CI,8CI);Propanedioic acid, bromo-, diethylester (9CI);2-Bromomalonic acid diethyl ester;Bromomalonic acid diethylester;Diethyl 2-bromo-1,3-propanedioate;Diethyl 2-bromomalonate;Diethyl2-bromopropanedioate;Diethyl bromomalonate;Diethyl a-bromomalonate;Ethylbromomalonate;NSC 1985;a-Bromomalonic ester;
- PSA 52.60000
- LogP 0.87610
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane for 1h; Heating; Irradiation; | 100% |
| Stage #1: diethyl malonate With sodium hydride In dimethyl sulfoxide at 40℃; for 2.5h; Stage #2: With copper(ll) bromide In dimethyl sulfoxide at 40℃; for 1.5h; | 99% |
| With Cl(CF2)4SO2Br at 10℃; for 10h; | 98% |

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Ambient temperature; | A 95% B 71% |


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 28℃; for 18h; Irradiation; Inert atmosphere; | 91% |
| With stannous fluoride In methanol |

| Conditions | Yield |
|---|---|
| With bromine at 20 - 25℃; for 3h; | A 4% B 87% |
-
-
631-22-1
diethyl dibromomalonate

-
A
-
599-99-5
ethyl tribromoacetate

-
B
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| With sodium methylate In tetrahydrofuran | A 75% B n/a |
-
-
52287-56-6
(E)-1-methyl-4-(2-nitroprop-1-en-1-yl)benzene

-
A
-
6174-95-4
1,1,2,2-tetracarboethoxy-ethylene

-
B
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran for 10h; Ambient temperature; | A 40% B 50% |
-
-
506-68-3
bromocyane

-
-
121-44-8
triethylamine

-
-
105-53-3
diethyl malonate

-
A
-
617-83-4
Diethylcyanamide

-
B
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| In acetone at 20℃; | A n/a B 38% |
| In acetone at 20℃; von Braun Amine Degradation; |


| Conditions | Yield |
|---|---|
| at 100 - 110℃; | |
| at 100 - 110℃; |
-
-
6186-89-6
ethyl methyl malonate

-
A
-
685-87-0
Diethyl 2-bromomalonate

-
B
-
93612-48-7
ethyl methyl bromomalonate

-
C
-
868-26-8
dimethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane for 0.5h; Heating; | A n/a B 12.1 g C n/a |


| Conditions | Yield |
|---|---|
| With tetrachloromethane; dibenzoyl peroxide |

-
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| With bromine |

| Conditions | Yield |
|---|---|
| With iron pentacarbonyl; water In benzene at 80℃; | A 40 % Chromat. B 14 % Chromat. |

| Conditions | Yield |
|---|---|
| at 20℃; for 12h; | 100% |
| In dichloromethane |
-
-
53510-88-6
potassium phthalimide-15N

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
5680-62-6
diethyl <15N>phthalimidomalonate

| Conditions | Yield |
|---|---|
| cetyltributylphosphonium bromide In toluene for 20h; | 100% |
-
-
84911-17-1
Ethyl 5-Thioxopyrrolidine-2-carboxylate

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
84911-19-3
Diethyl 2-<2-(Ethoxycarbonyl)-5-pyrrolidinylidene>malonate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate Eschenmoser coupling reaction; | 100% |
| With sodium hydrogencarbonate In dichloromethane for 24h; Heating; | 42% |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
226903-40-8
1-[[(Z)-6-ethoxy-4-methylene-5-hexenyl]oxy]-4-methoxybenzene

-
-
226903-45-3
diethyl 6-ethoxy-3,6-dihydro-4-[3-(p-methoxyphenoxy)propyl]-2H-thiopyran-2,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With sulfur; triethylamine In acetonitrile at 20℃; for 27h; Cycloaddition; | 100% |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
80442-95-1
ethyl (2S)-5-thioxo-3,4-dihydro-5H-pyrrole-2-carboxylate

-
-
675588-09-7
(S)-5-(bis(ethoxycarbonyl)methylidene)pyrrolidine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane for 48h; Eschenmoser contraction; Heating; | 100% |
| With sodium carbonate In dichloromethane for 2.5h; Eschenmoser Sulfide Contraction; Sonication; | 73% |

-
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium carbonate In water; N,N-dimethyl-formamide for 22h; | 100% |

| Conditions | Yield |
|---|---|
| With C18H13NOS; N-ethyl-N,N-diisopropylamine In acetonitrile for 1.66667h; Irradiation; | 100% |
| With 2,6-dimethylpyridine for 24h; Irradiation; Inert atmosphere; | 87% |
| With N,N-diphenylaminobenzene; 5,5'-bis((2-(trifluoromethyl)phenyl)ethynyl)-2,2'-bithiophene In N,N-dimethyl-formamide at 20℃; for 0.666667h; Irradiation; | 40 mg |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
14064-10-9
diethyl 2-chloromalonate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In dimethyl sulfoxide at 20℃; for 24h; | 99.4% |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
88-75-5
2-hydroxynitrobenzene

-
-
32539-24-5
diethyl 2-(2'-nitrophenoxy)malonate

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxynitrobenzene With potassium hydroxide In ethanol for 0.5h; Stage #2: Diethyl 2-bromomalonate In N,N-dimethyl-formamide for 16h; Inert atmosphere; | 99% |
| With potassium fluoride In N,N-dimethyl-formamide at 60℃; for 6h; Substitution; | 70% |
-
-
15128-82-2
3-hydroxy-2-nitropyridine

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
499787-33-6
diethyl 2-[(2-nitro-3-pyridyl)oxy]malonate

| Conditions | Yield |
|---|---|
| Stage #1: 3-hydroxy-2-nitropyridine With potassium fluoride In N,N-dimethyl-formamide at 0℃; for 0.5h; Kikelj reaction; Stage #2: Diethyl 2-bromomalonate In N,N-dimethyl-formamide at 20℃; for 24h; | 99% |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
81289-78-3
diethyl bromochloromalonate

| Conditions | Yield |
|---|---|
| With sodium hypochlorite In acetic acid; acetone at 0℃; for 1h; | 99% |
| With sodium hypochlorite; acetic acid In acetone at 0℃; | 98% |
| Multi-step reaction with 2 steps 1: N-chloro-succinimide / dimethyl sulfoxide / 24 h / 20 °C 2: acetic acid; sodium hypobromide / acetone / 12 h / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 2-nitro-4-fluorophenol With potassium hydroxide In ethanol for 1h; Stage #2: Diethyl 2-bromomalonate In N,N-dimethyl-formamide for 24h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-6-nitrophenol With potassium hydroxide In ethanol for 1h; Stage #2: Diethyl 2-bromomalonate In N,N-dimethyl-formamide for 24h; Inert atmosphere; | 99% |
-
-
85-19-8
(5-chloro-2-hydroxyphenyl)phenylmethanone

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
27006-00-4
ethyl 5-chloro-3-phenyl-1-benzofuran-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (5-chloro-2-hydroxyphenyl)phenylmethanone With potassium carbonate In acetone for 0.5h; Inert atmosphere; Stage #2: Diethyl 2-bromomalonate In acetone Inert atmosphere; Reflux; | 99% |
-
-
61785-35-1
SL 81.0196

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
1263152-60-8
ethyl 5-chloro-3-(2-chlorophenyl)-1-benzofuran-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: SL 81.0196 With potassium carbonate In acetone for 0.5h; Inert atmosphere; Stage #2: Diethyl 2-bromomalonate In acetone Inert atmosphere; Reflux; | 99% |
-
-
821-41-0
5-Hexen-1-ol

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
1277180-98-9
diethyl 2-(2-bromo-6-hydroxyhexyl)malonate

| Conditions | Yield |
|---|---|
| With Ir[(dF(CF3)ppy)2(dtbbpy)]PF6; lithium bromide In water; N,N-dimethyl-formamide for 24h; Visible-light irradiation; Inert atmosphere; | 99% |
| With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; lithium bromide In water; N,N-dimethyl-formamide Inert atmosphere; Irradiation; | 99% |
| With 2,6-dimethylpyridine; 4-methoxy-benzaldehyde In acetonitrile at 25℃; for 26h; Schlenk technique; Inert atmosphere; Sealed tube; Irradiation; | 99% |
-
-
25118-23-4
ethyl 6-heptenoate

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
1277181-03-9
triethyl 3-bromoheptane-1,1,7-tricarboxylate

| Conditions | Yield |
|---|---|
| With Ir[(dF(CF3)ppy)2(dtbbpy)]PF6; lithium bromide In water; N,N-dimethyl-formamide for 24h; Visible-light irradiation; Inert atmosphere; | 99% |
-
-
78888-18-3
N-tert-butoxycarbonylprop-2-en-1-amine

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
1277180-97-8
diethyl 2-(2-bromo-3-((tert-butoxycarbonyl)amino)propyl)malonate

| Conditions | Yield |
|---|---|
| With Ir[(dF(CF3)ppy)2(dtbbpy)]PF6; lithium bromide In water; N,N-dimethyl-formamide for 24h; Visible-light irradiation; Inert atmosphere; | 99% |
| With C30H26N6Ru(2+)*2Cl(1-); lithium bromide In dimethyl sulfoxide for 24h; Inert atmosphere; Irradiation; | 99% |
| With C44H29CuN4O3P(1+)*BF4(1-); lithium bromide In water; N,N-dimethyl-formamide at 20℃; for 20h; Kinetics; Reagent/catalyst; UV-irradiation; | 89% |

| Conditions | Yield |
|---|---|
| With [Ir(ppy)2(dtbbpy)]PF6; triethylamine In dichloromethane at 20℃; under 760.051 Torr; Inert atmosphere; Irradiation; | 99% |

-
-
64-17-5
ethanol

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
260967-14-4
benzyl N-[(1E)-prop-1-en-1-yl]-carbamate

| Conditions | Yield |
|---|---|
| With [Ir(ppy)2(dtbbpy)]PF6; triethylamine In dichloromethane at 20℃; under 760.051 Torr; for 1h; Inert atmosphere; sunlight; optical yield given as %de; | 99% |

-
-
13707-46-5
N-tosyl-4-nitrobenzaldimine

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
1342891-82-0
diethyl 3-(4-nitrophenyl)-1-tosylaziridine-2,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In acetonitrile; mineral oil at 0 - 20℃; Inert atmosphere; | 99% |
| With sodium hydride In acetonitrile for 0.283333h; Inert atmosphere; | 84% |
| With sodium hydride In acetonitrile; mineral oil at 0 - 20℃; Inert atmosphere; |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
143206-01-3
N-(4-cyanobenzylidene)-p-toluenesulfonamide

-
-
1132667-73-2
diethyl 3-(4-cyanophenyl)-1-tosylaziridine-2,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In acetonitrile; mineral oil at 0 - 20℃; Inert atmosphere; | 99% |
| With sodium hydride In acetonitrile for 0.233333h; Inert atmosphere; | 90% |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
163926-77-0
4-methyl-N-[propylidene]benzene-1-sulfonamide

-
-
1373511-46-6
diethyl 3-ethyl-1-tosylaziridine-2,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In acetonitrile; mineral oil at 0 - 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxy-benzylamine With potassium carbonate In acetonitrile at 20℃; for 2h; Reflux; Stage #2: Diethyl 2-bromomalonate In acetonitrile at 20℃; | 99% |
| With triethylamine In chloroform Reflux; | 20% |
-
-
104-53-0
3-phenyl-propionaldehyde

-
-
685-87-0
Diethyl 2-bromomalonate

-
-
1085512-00-0
(R)-diethyl 2-(1-oxo-3-phenylpropan-2-yl)malonate

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; C21H22N2O2S In N,N-dimethyl-formamide at 15℃; for 18h; Catalytic behavior; Quantum yield; Reagent/catalyst; Solvent; Irradiation; enantioselective reaction; | 99% |
| With 2,6-dimethylpyridine; C11H12ClNOPtS2; C14H20N2O In N,N-dimethyl-formamide at 20℃; Inert atmosphere; Irradiation; enantioselective reaction; | 91% |
| With 2,6-dimethylpyridine; (2R,5S)-2-tert-butyl-3,5-dimethylimidazolidin-4-one hydrogen chloride; bismuth(III) oxide In N,N-dimethyl-formamide at 20℃; for 1h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Sealed tube; Irradiation; enantioselective reaction; | 86% |
| With 2,6-dimethylpyridine; (2R,5S)-2-tert-butyl-3,5-dimethylimidazolidin-4-one trifluoromethanesulfonate In N,N-dimethyl-formamide for 24h; Catalytic behavior; Reagent/catalyst; Solvent; Inert atmosphere; Irradiation; Sealed tube; enantioselective reaction; | 83% |
| With 2,6-dimethylpyridine; (2R,5S)-2-tert-butyl-3,5-dimethylimidazolidin-4-one trifluoromethanesulfonate; [Fe(bpy)3]Br2 In N,N-dimethyl-formamide at 25℃; for 16h; Irradiation; enantioselective reaction; | 78% |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
99802-91-2
2-cinnamoyl-1-methyl-1H-imidazole

| Conditions | Yield |
|---|---|
| Stage #1: 2-cinnamoyl-1-methyl-1H-imidazole With C38H38N4O2Rh(1+)*BF4(1-) In chloroform at 20℃; for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: Diethyl 2-bromomalonate With triethylamine In chloroform at 20℃; for 3h; Reagent/catalyst; Solvent; Schlenk technique; Inert atmosphere; enantioselective reaction; | 99% |

-
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| Stage #1: C18H14N2O With C38H38N4O2Rh(1+)*BF4(1-) In chloroform at 20℃; for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: Diethyl 2-bromomalonate With triethylamine In chloroform at 20℃; for 10h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 99% |

-
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| Stage #1: C14H14N2O With C38H38N4O2Rh(1+)*BF4(1-) In chloroform at 20℃; for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: Diethyl 2-bromomalonate With triethylamine In chloroform at 20℃; for 10h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 99% |

-
-
685-87-0
Diethyl 2-bromomalonate

| Conditions | Yield |
|---|---|
| Stage #1: C14H14N2O2 With C38H38N4O2Rh(1+)*BF4(1-) In chloroform at 20℃; for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: Diethyl 2-bromomalonate With triethylamine In chloroform at 20℃; for 24h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 99% |
Diethyl bromomalonate Chemical Properties
Molecule structure of Diethyl bromomalonate (CAS NO.685-87-0):
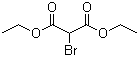
IUPAC Name: Diethyl 2-bromopropanedioate
Molecular Weight: 239.06384 [g/mol]
Molecular Formula: C7H11BrO4
Index of Refraction: 1.467
Molar Refractivity: 45.71 cm3
Molar Volume: 164.6 cm3
Surface Tension: 38.3 dyne/cm
Density: 1.452 g/cm3
Flash Point: 89.6 °C
Enthalpy of Vaporization: 47.07 kJ/mol
Boiling Point: 234 °C at 760 mmHg
Vapour Pressure: 0.0542 mmHg at 25 °C
Sensitive: lachrymatory
XLogP3-AA: 1.8
H-Bond Acceptor: 4
Rotatable Bond Count: 6
Exact Mass: 237.984071
MonoIsotopic Mass: 237.984071
Topological Polar Surface Area: 52.6
Heavy Atom Count: 12
Canonical SMILES: CCOC(=O)C(C(=O)OCC)Br
InChI: InChI=1S/C7H11BrO4/c1-3-11-6(9)5(8)7(10)12-4-2/h5H,3-4H2,1-2H3
InChIKey: FNJVDWXUKLTFFL-UHFFFAOYSA-N
EINECS: 211-683-1
Product Categories: C6 to C7; Carbonyl Compounds; Esters
Diethyl bromomalonate Safety Profile
Hazard Codes:  C
C
Risk Statements: 34
R34:Causes burns.
Safety Statements: 26-36/37/39-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 3265 8/PG 2
WGK Germany: 3
F: 19
Hazard Note: Corrosive/Lachrymator
HazardClass: 8
PackingGroup: III
Diethyl bromomalonate Specification
Diethyl bromomalonate (CAS NO.685-87-0) is also named as AI3-01376 ; Bromomalonic acid diethyl ester ; Diethyl 2-bromomalonate ; Ethyl bromomalonate ; Malonic acid, bromo-, diethyl ester ; NSC 1985 ; alpha-Bromomalonic ester ; Propanedioic acid, 2-bromo-, 1,3-diethyl ester ; Propanedioic acid, bromo-, diethyl ester . Diethyl bromomalonate (CAS NO.685-87-0) is clear colourless to slightly yellow liquid.
Related Products
- Diethyl (1-phenylpropyl)malonate
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(diethoxymethylsilyl)ethyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (beta,gamma-epoxypropyl)phosphonate
- 685-88-1
- 68588-39-6
- 685892-09-5
- 685898-44-6
- 685899-12-1
- 685899-13-2
- 6859-01-4
- 685902-89-0
- 68591-34-4
- 685-91-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View