-
Name
Dilthiazem hydrochloride
- EINECS 251-443-3
- CAS No. 33286-22-5
- Article Data11
- CAS DataBase
- Density 1.26g/cm3
- Solubility Soluble in water
- Melting Point 212-214 ºC
- Formula C22H26N2O4S.HCl
- Boiling Point 594.4 ºC at 760 mmHg
- Molecular Weight 450.986
- Flash Point 313.3 ºC
- Transport Information UN 3249
- Appearance Fine needles
- Safety 36-45-36/37-26
- Risk Codes 22-40-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 1,5-Benzothiazepin-4(5H)-one,3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-,monohydrochloride, (2S,3S)- (9CI);1,5-Benzothiazepin-4(5H)-one,3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-,monohydrochloride, (2S-cis)-;(+)-Diltiazem hydrochloride;(2S,3S)-(+)-cis-Diltiazem hydrochloride;(S,S)-Diltiazem hydrochloride;Adizem;Altiazem;Anginyl;Blocalcin 60;Britiazim;CRD401;Calcicard;Cardizem SR;Dilacor XR;Diladel;Dilcardia;Dilgard;Dilzene;d-cis-3-Acetoxy-2,3-dihydro-5-[2-(dimethylamino)ethyl]-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5H)-onehydrochloride;d-cis-Diltiazem hydrochloride;
- PSA 84.38000
- LogP 4.23550
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: O-desacetyldiltiazem; acetic anhydride With dmap; triethylamine In dichloromethane for 3h; Heating / reflux; Stage #2: With hydrogenchloride In methanol pH=2; | 92% |
| With hydrogenchloride In ethanol; butanone at 90℃; for 1h; | 80% |
| With dmap In dichloromethane for 3h; Heating; Yield given; |
-
-
75472-91-2
(2S,3S)-5-(2-dimethylaminoethyl)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one hydrochloride

-
-
108-24-7
acetic anhydride

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3S)-5-(2-dimethylaminoethyl)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one hydrochloride; acetic anhydride With dmap; triethylamine In dichloromethane Stage #2: With hydrogenchloride In methanol pH=2; | 92% |
| With pyridine; dmap |
-
-
108-22-5
Isopropenyl acetate

-
-
75472-91-2
(2S,3S)-5-(2-dimethylaminoethyl)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one hydrochloride

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In chloroform for 0.75h; Heating; | 88.8% |
-
-
2419-67-2, 24470-83-5, 41564-61-8
(E)-1-(4-methoxyphenyl)-4,4-dimethylpent-1-en-3-one

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 70 percent / urea hydrogen peroxide, DBU, immobilised poly-L-leucine / tetrahydrofuran / 28 h 2: 84 percent / MCPBA, KF / CH2Cl2 / 24 h 3: 90 percent / toluene / 17 h / Heating 4: 94 percent / xylene / 216 h / Heating 5: K2CO3 / ethyl acetate 6: pyridine, DMAP View Scheme |
-
-
42399-49-5
(2S,3S)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3 / ethyl acetate 2: pyridine, DMAP View Scheme | |
| Multi-step reaction with 2 steps 1: 67 percent / NaH / dimethylformamide; paraffin; diethyl ether / 3 h / 70 °C 2: 80 percent / 2.) HCl / butan-2-one; ethanol / 1 h / 90 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 88.2 percent / K2CO3 / H2O; ethyl acetate / 5 h / Heating 2: DMAP / CH2Cl2 / 3 h / Heating View Scheme |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 87 percent / NaOMe / methanol / 40 h / Heating 2: 70 percent / urea hydrogen peroxide, DBU, immobilised poly-L-leucine / tetrahydrofuran / 28 h 3: 84 percent / MCPBA, KF / CH2Cl2 / 24 h 4: 90 percent / toluene / 17 h / Heating 5: 94 percent / xylene / 216 h / Heating 6: K2CO3 / ethyl acetate 7: pyridine, DMAP View Scheme |
-
-
201804-22-0
tert-butyl (2R,3S)-3-(4-methoxyphenyl)glycidate

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / toluene / 17 h / Heating 2: 94 percent / xylene / 216 h / Heating 3: K2CO3 / ethyl acetate 4: pyridine, DMAP View Scheme |
-
-
201804-23-1
(2S,3S)-3-(2-Amino-phenylsulfanyl)-2-hydroxy-3-(4-methoxy-phenyl)-propionic acid tert-butyl ester

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 94 percent / xylene / 216 h / Heating 2: K2CO3 / ethyl acetate 3: pyridine, DMAP View Scheme |
-
-
201804-19-5
1-((2R,3S)-4-methoxyphenyloxiranyl)-2,2-dimethylpropan-1-one

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 84 percent / MCPBA, KF / CH2Cl2 / 24 h 2: 90 percent / toluene / 17 h / Heating 3: 94 percent / xylene / 216 h / Heating 4: K2CO3 / ethyl acetate 5: pyridine, DMAP View Scheme |
-
-
42399-48-4
(2S,3S)-threo-2-hydroxy-3-(2-aminophenylthio)-3-(4-methoxyphenyl)-propionic acid

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 88 percent / PTSA / xylene / 16 h / Heating 2: 88.2 percent / K2CO3 / H2O; ethyl acetate / 5 h / Heating 3: DMAP / CH2Cl2 / 3 h / Heating View Scheme |
-
-
138382-02-2
(αS,βS,1R,2S)-β-<(2-aminophenyl)thio>-α-hydroxy-β-(4-methoxyphenyl)propanoic acid 2-phenylcyclohexyl ester hydrochloride

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 81 percent / 2 N NaOH / ethanol; H2O / 2 h / Heating 2: 88 percent / PTSA / xylene / 16 h / Heating 3: 88.2 percent / K2CO3 / H2O; ethyl acetate / 5 h / Heating 4: DMAP / CH2Cl2 / 3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 73.2 percent / PTSA / xylene / 16 h / Heating 2: 88.2 percent / K2CO3 / H2O; ethyl acetate / 5 h / Heating 3: DMAP / CH2Cl2 / 3 h / Heating View Scheme |
-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide at 100℃; for 0.5h; | 96% |
-
-
33286-22-5
diltiazem hydrochloride

-
-
23515-44-8
cis(+)-3-hydroxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one

| Conditions | Yield |
|---|---|
| With lithium hydroxide; water In tetrahydrofuran at 160℃; for 0.0833333h; Microwave irradiation; | 94% |
-
-
33286-22-5
diltiazem hydrochloride

-
-
42399-40-6
O-desacetyldiltiazem

| Conditions | Yield |
|---|---|
| In water Ambient temperature; Irradiation; decomposition of diltiazem in various solvents; | |
| With sodium chloride at 79.85℃; Kinetics; Thermodynamic data; Activation energy; Further Variations:; Temperatures; relative humidity; | |
| With hydrogenchloride at 39.85℃; pH=0.43; Kinetics; Further Variations:; pH-values; Reagents; Temperatures; |
-
-
33286-22-5
diltiazem hydrochloride

-
-
42399-41-7
diltiazem

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; ethyl acetate | |
| With sodium carbonate In water pH=7.5; | |
| With sodium hydrogencarbonate In water for 0.25h; |

-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer pH=7.4; UV-irradiation; |
-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium carbonate / water / pH 7.5 2: diethyl ether / 20 °C View Scheme |
-
-
33286-22-5
diltiazem hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium carbonate / water / pH 7.5 2: acetonitrile / 20 °C View Scheme |
Dilthiazem hydrochloride Specification
The Diltiazem HCl, with the CAS registry number 33286-22-5, is also known as (+)-cis-3-(Acetyloxy)-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)one monohydrochloride. It belongs to the product categories of Active Pharmaceutical Ingredients; Intermediates & Fine Chemicals; Pharmaceuticals; Calcium Channel; Ion Channels; Aromatics; Chiral Reagents; Sulfur & Selenium Compounds. Its EINECS registry number is 251-443-3. This chemical's molecular formula is C22H27ClN2O4S and molecular weight is 450.98. What's more, both its IUPAC name and systematic name are the same which is called [(2S,3S)-5-[2-(Dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] acetate hydrochloride. It is used as a calcium channel blocher with vasodilating activity and an antianginal, antihypertensive.
Physical properties about Diltiazem HCl are: (1)ACD/LogP: 3.63; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.76; (4)ACD/LogD (pH 7.4): 2.26; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 14.49; (7)ACD/KOC (pH 5.5): 3.03; (8)ACD/KOC (pH 7.4): 96.24; (9)#H bond acceptors: 6; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 7; (12)Polar Surface Area: 84.38 Å2; (13)Flash Point: 313.3 °C; (14)Enthalpy of Vaporization: 88.6 kJ/mol; (15)Boiling Point: 594.4 °C at 760 mmHg; (16)Vapour Pressure: 4.27E-14 mmHg at 25 °C.
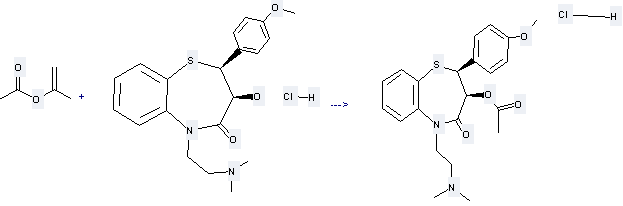
Preparation of Diltiazem HCl: this chemical can be prepared by 2-Acetoxy-propene with (2S,3S)-5-(2-Dimethylaminoethyl)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one hydrochloride. This reaction needs reagent methanesulfonic acid, solvent CHCl3 and other condition of heating for 45 min. The yield is 88.8 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. If swallowed, it's harmful to health. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1) SMILES: Cl.O=C2N(c3c(S[C@@H](c1ccc(OC)cc1)[C@H]2OC(=O)C)cccc3)CCN(C)C
(2) InChI: InChI=1S/C22H26N2O4S.ClH/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3;/h5-12,20-21H,13-14H2,1-4H3;1H/t20-,21+;/m1./s1
(3) InChIKey: HDRXZJPWHTXQRI-BHDTVMLSSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | 40mg/kg (40mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD GASTROINTESTINAL: NAUSEA OR VOMITING | Kiso to Rinsho. Clinical Report. Vol. 21, Pg. 4843, 1987. |
| man | LDLo | oral | 21mg/kg (21mg/kg) | CARDIAC: CARDIOMYOPATHY INCLUDING INFARCTION CARDIAC: PULSE RATE VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION | Postgraduate Medical Journal. Vol. 69, Pg. 474, 1993. |
| man | TDLo | oral | 3429ug/kg/2D- (3.429mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Lancet. Vol. 341, Pg. 967, 1993. |
| man | TDLo | oral | 15420ug/kg/7D (15.42mg/kg) | VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION | Archives of Internal Medicine. Vol. 151, Pg. 1869, 1991. |
| man | TDLo | oral | 36mg/kg/13D-I (36mg/kg) | LIVER: "HEPATITIS, FIBROUS (CIRRHOSIS, POST-NECROTIC SCARRING)" | Gastroenterology. Vol. 88, Pg. 1260, 1985. |
| mouse | LD50 | intraperitoneal | 177mg/kg (177mg/kg) | Journal of Medicinal Chemistry. Vol. 29, Pg. 820, 1986. | |
| mouse | LD50 | intravenous | 58mg/kg (58mg/kg) | BEHAVIORAL: ANTIPSYCHOTIC | Japanese Journal of Pharmacology. Vol. 22, Pg. 467, 1972. |
| mouse | LD50 | oral | 508mg/kg (508mg/kg) | Journal of Medicinal Chemistry. Vol. 33, Pg. 2192, 1990. | |
| mouse | LD50 | subcutaneous | 260mg/kg (260mg/kg) | BEHAVIORAL: ANTIPSYCHOTIC | Japanese Journal of Pharmacology. Vol. 22, Pg. 467, 1972. |
| rat | LD50 | intravenous | 38mg/kg (38mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TETANY | Japan Medical Gazette. Vol. 11(1), Pg. 12, 1974. |
| rat | LD50 | oral | 560mg/kg (560mg/kg) | BEHAVIORAL: ANTIPSYCHOTIC | Japanese Journal of Pharmacology. Vol. 22, Pg. 467, 1972. |
| rat | LD50 | subcutaneous | 520mg/kg (520mg/kg) | BEHAVIORAL: ANTIPSYCHOTIC | Japanese Journal of Pharmacology. Vol. 22, Pg. 467, 1972. |
| women | LDLo | oral | 120mg/kg (120mg/kg) | CARDIAC: CARDIOMYOPATHY INCLUDING INFARCTION | Human & Experimental Toxicology. Vol. 13, Pg. 161, 1994. |
| women | TDLo | oral | 7200ug/kg/2D- (7.2mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: TOXIC PSYCHOSIS | Journal of the Royal Society of Medicine. Vol. 81, Pg. 296, 1988. |
| women | TDLo | oral | 7200ug/kg (7.2mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: TOXIC PSYCHOSIS | Journal of the Royal Society of Medicine. Vol. 81, Pg. 296, 1988. |
| women | TDLo | oral | 8400ug/kg (8.4mg/kg) | CARDIAC: PULSE RATE VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION | Annals of Emergency Medicine. Vol. 22, Pg. 196, 1993. |
| women | TDLo | oral | 18mg/kg (18mg/kg) | CARDIAC: CARDIOMYOPATHY INCLUDING INFARCTION CARDIAC: PULSE RATE VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION | Journal of Toxicology, Clinical Toxicology. Vol. 29, Pg. 45, 1991. |
| women | TDLo | oral | 18mg/kg (18mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION SKIN AND APPENDAGES (SKIN): SWEATING: OTHER | Journal of Toxicology, Clinical Toxicology. Vol. 29, Pg. 45, 1991. |
| women | TDLo | oral | 19mg/kg (19mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Postgraduate Medical Journal. Vol. 64, Pg. 467, 1988. |
Related Products
- Dilthiazem hydrochloride
- 3328-68-5
- 332884-35-2
- 33288-71-0
- 3328-88-9
- 33289-76-8
- 33290-12-9
- 332903-74-9
- 332927-03-4
- 33296-21-8
- 33300-72-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View