-
Name
Dimethyl fumarate
- EINECS 210-849-0
- CAS No. 624-49-7
- Article Data218
- CAS DataBase
- Density 1.124 g/cm3
- Solubility Soluble in water (1.6 mg/ml at 20°C), methanol (30-36 mg/ml), ethanol (10 mg/ml at 25°C), DMSO (29 mg/ml at 25°C), and DMF (~12 mg/ml).
- Melting Point 102-106 °C(lit.)
- Formula C6H8O4
- Boiling Point 193 °C at 760 mmHg
- Molecular Weight 144.127
- Flash Point 91.1 °C
- Transport Information
- Appearance fine white crystalline powder
- Safety 26-36/37/39
- Risk Codes 21-38-41-43-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2-Butenedioic acid (E)-, dimethyl ester;Fumaric acid, dimethyl ester (6CI,8CI);(2E)-2-Butenedioic acid dimethyl ester;(E)-But-2-enedioic acid dimethyl ester;AZL-O 211089;Dimethyl (E)-2-butenedioate;Dimethyl(E)-butenedioate;2-Butenedioic acid(2E)-, 1,4-dimethyl ester;Dimethyl trans-ethylenedicarboxylate;NSC 167432;trans-1,2-Ethylenedicarboxylic aciddimethyl ester;trans-Butenedioic acid dimethyl ester;
- PSA 52.60000
- LogP -0.11140
Synthetic route

| Conditions | Yield |
|---|---|
| With 1-hexylimidazolium DL-lactate at 70℃; for 24h; Kinetics; Product distribution; Further Variations:; Reagents; Temperatures; | 100% |
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane Heating; | 100% |
| With bromine In dichloromethane at 50℃; for 5h; Irradiation; | 98% |
-
-
117753-93-2
(P(CH2CH2P(C6H5)2)3)Co(H2)(1+)*PF6(1-) = (P(CH2CH2P(C6H5)2)3)CoH2PF6

-
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 100% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 8h; Reflux; | 99% |
| With acetyl chloride at 45 - 60℃; for 5.5h; Temperature; Reagent/catalyst; | 99.85% |
| With sulfuric acid at 105℃; for 0.266667h; Temperature; | 99% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 55℃; for 2h; | 98.6% |
| With thionyl chloride at 10 - 65℃; | 80 g |

| Conditions | Yield |
|---|---|
| With sulfuric acid Reflux; regioselective reaction; | 96% |
| With perchloric acid for 18h; Reflux; | 9.3 g |
-
-
112193-23-4
(4R,5R)-2-methoxy-[1,3]dioxolane-4,5-dicarboxylic acid dimethyl ester

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In acetic anhydride for 4h; Heating; | 95% |

-
A
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
1058-14-6
N-p-tolylsulfonylphosphine imide

-
C
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In toluene for 14h; Heating; | A n/a B 93% C n/a |

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
110-17-8
(2E)-but-2-enedioic acid

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; Reflux; | 93% |

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With lithium iodide In acetone for 0.166667h; Ambient temperature; | 90% |
| With magnesium iodide In acetonitrile for 0.0833333h; Ambient temperature; | 90% |
| With iodine; triphenylphosphine In dichloromethane for 2h; Ambient temperature; various conditions; | 85% |
-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
-
74-86-2
acetylene

-
A
-
692-91-1, 692-92-2, 1119-43-3, 1733-37-5
cis,cis-dimethyl muconate

-
B
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
C
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| PdI2-KI at 60℃; under 19000 Torr; for 15h; | A 7% B 89% C 3% |

-
-
623-43-8, 4358-59-2, 18707-60-3, 130981-57-6
methyl crotonate

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane for 3h; Inert atmosphere; Reflux; | A 89% B n/a |


-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With iodine; triphenylphosphine In dichloromethane Ambient temperature; | 86% |
| With triphenylphosphine In xylene at 110℃; | 45% |
-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
-
74-86-2
acetylene

-
A
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; oxygen; copper dichloride; palladium dichloride under 1 Torr; for 2h; | A 86% B 14% |

-
-
6832-16-2
methyl diazoacetate

-
-
55261-56-8
3-morpholino-but-2-enoic acid methyl ester

-
A
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| copper acetylacetonate In ethyl acetate Heating; | A n/a B n/a C 86% |


| Conditions | Yield |
|---|---|
| With diethylamino-sulfur trifluoride In chloroform at 0℃; for 0.5h; | A 85% B 6% |
-
-
132559-56-9
3-<1.2.4>trioxolan-3-ylmethyl-cyclohexanone

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
A
-
132559-62-7
4-(3-oxocyclohexyl)-2(E)-butenoic acid methyl ester

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 12h; Wittig type reaction; | A 85% B 3% |

-
-
23652-59-7
4-(pyrrolidin-1-yl)pent-3-en-2-one

-
-
6832-16-2
methyl diazoacetate

-
A
-
85392-47-8
methyl 2-acetyl-4-oxovalerate

-
B
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
C
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| copper(I) triflate In dichloromethane at 20℃; for 24h; | A 84% B n/a C n/a |

-
-
6832-16-2
methyl diazoacetate

-
-
137201-66-2
E-methyl β-morpholino-cinnamate

-
A
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| copper acetylacetonate In ethyl acetate Heating; | A n/a B n/a C 84% |

-
-
6832-16-2
methyl diazoacetate

-
A
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| copper acetylacetonate In ethyl acetate Heating; | A n/a B n/a C 83% |

-
-
608-68-4, 608-69-5, 5057-96-5, 13171-64-7, 117356-23-7
dimethyl L-tartrate

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With 1H-imidazole; iodine; chloro-diphenylphosphine In toluene; acetonitrile Ambient temperature; | 81% |
-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
A
-
3878-44-2
Triphenylphosphine selenide

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With selenium In toluene for 3h; Heating; | A 77.6% B 73.6% |
-
-
762-42-5
dimethyl acetylenedicarboxylate

-
A
-
89330-93-8
dimethyl phenylmaleate

-
B
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
C
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With cyclopentadienylchromiumtricarbonyl hydride In benzene Inert atmosphere; | A 76% B 17% C 5% |

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With selenium In toluene for 4h; Heating; | 74% |

| Conditions | Yield |
|---|---|
| sulfuric acid In methanol Heating; | 73% |
-
-
70008-80-9
3-morpholin-4-yl-1-phenyl-but-2-en-1-one

-
-
6832-16-2
methyl diazoacetate

-
A
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
75519-84-5
3-oxo-2-(2-oxo-2-phenyl-ethyl)-butyric acid methyl ester

-
C
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| copper(I) triflate In dichloromethane at 20℃; | A n/a B 73% C n/a D 4% |

-
-
489-84-9
7-isopropyl-1,4-dimethyl-azulene

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
A
-
98525-07-6, 72898-39-6, 98525-12-3, 98525-13-4
dimethyl 1,6-dimethyl-9-(1-methylethyl)heptalene-4,5-dicarboxylate

-
B
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
C
-
145948-88-5
dimethyl (E)-1-(5'-isopropyl-3',8'-dimethylazulen-1-yl)-ethene-1,2-dicarboxylate

-
D
-
145948-89-6
dimethyl (Z)-1-(5-isopropyl-3,8-dimethylazulen-1-yl)ethene-1,2-dicarboxylate

-
E
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In acetonitrile at 100℃; for 4h; Mechanism; other solvents, other substrates; | A 71% B 1% C 15% D 11% E 1% |

-
-
489-84-9
7-isopropyl-1,4-dimethyl-azulene

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
A
-
98525-07-6, 72898-39-6, 98525-12-3, 98525-13-4
dimethyl 1,6-dimethyl-9-(1-methylethyl)heptalene-4,5-dicarboxylate

-
B
-
145948-88-5
dimethyl (E)-1-(5'-isopropyl-3',8'-dimethylazulen-1-yl)-ethene-1,2-dicarboxylate

-
C
-
145948-89-6
dimethyl (Z)-1-(5-isopropyl-3,8-dimethylazulen-1-yl)ethene-1,2-dicarboxylate

-
D
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In acetonitrile at 100℃; for 4h; Further byproducts given; | A 71% B 15% C 11% D 1% |

-
-
67-56-1
methanol

-
-
110-17-8
(2E)-but-2-enedioic acid

-
A
-
2756-87-8
methyl hydrogen fumarate

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| sulfuric acid at 60 - 70℃; for 3h; Product distribution / selectivity; Industry scale; | A n/a B 71% |

-
-
67866-50-6
diethyl (2-bromo-1-phenylvinyl)phosphonite

-
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| for 4h; Ambient temperature; | A 27% B 70% |

-
-
70008-81-0
(E)-3-morpholin-4-yl-1,3-diphenylpropenone

-
-
6832-16-2
methyl diazoacetate

-
A
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
B
-
90904-37-3
methyl α,β-dibenzoyl-propionate

-
C
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| copper(I) triflate In dichloromethane at 20℃; | A n/a B 70% C n/a |

-
-
50-00-0
formaldehyd

-
-
17136-36-6
N-benzylglycine

-
-
624-49-7
dimethylfumarate

-
-
87813-05-6, 87813-06-7, 111186-67-5
dimethyl (3R,4R)-1-benzylpyrrolidine-3,4-dicarboxylate

| Conditions | Yield |
|---|---|
| In toluene for 0.25h; Heating; | 100% |
| In benzene for 3h; Heating; | 63% |
| In toluene |

-
-
109-69-3
n-Butyl chloride

-
-
90606-10-3
(E)-N-(phenylmethylene)-1-(trimethylsilyl)methylamine

-
-
624-49-7
dimethylfumarate

-
-
82959-82-8, 82959-83-9, 82959-84-0, 82959-85-1
(3R,4R)-2-Phenyl-pyrrolidine-3,4-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 40 - 45℃; | 100% |

-
-
57402-97-8
N-<(trimethylsilyl)methyl>benzaldimine

-
-
624-49-7
dimethylfumarate

-
-
82959-82-8, 82959-83-9, 82959-84-0, 82959-85-1
(3R,4S)-2-Phenyl-pyrrolidine-3,4-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With water; acetic acid In N,N,N,N,N,N-hexamethylphosphoric triamide for 24h; Ambient temperature; | 100% |
-
-
61201-25-0
3-methylene-2-phenyl-4-penten-2-ol

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In benzene for 16h; Heating; | 100% |
-
-
40448-79-1
isoquinolinium phenylacylide

-
-
624-49-7
dimethylfumarate

-
-
97204-11-0, 97275-44-0, 105017-20-7
(1R,2R,3R,10bR)-3-Benzoyl-1,2,3,10b-tetrahydro-pyrrolo[2,1-a]isoquinoline-1,2-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| In chloroform for 0.166667h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In cyclohexane at 25℃; Rate constant; | 100% |
| In tetrahydrofuran at -30℃; for 0.0833333h; | 100% |

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In chlorobenzene Heating; | 100% |
-
-
100281-17-2
2-(4-methoxyphenyl)-3-methylenepent-4-en-2-ol

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In benzene for 10h; Heating; | 100% |

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 0.166667h; Ambient temperature; | 100% |
-
-
23134-50-1
1H-2-naphtho<2,1-b>thiete

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In toluene at 80 - 110℃; | 100% |
| at 80 - 110℃; Yield given; |
-
-
162889-67-0
(diisopropoxyphosphinoyl)formonitriole oxide

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; | 100% |
-
-
174006-54-3
2H-naphtho<1,2-b>thiete

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In toluene at 80 - 110℃; | 100% |
| In toluene Heating; | 100% |
-
-
177028-67-0
2-oxo-4H-3,1-naptho<1,2-d>oxathiin

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In toluene for 16h; Heating; | 100% |
-
-
41078-15-3
3-aminothieno<3,4:3',4'>benzopyran-4-one

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| Heating; | 100% |
| at 170℃; for 4h; | 76% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In benzene at 80℃; for 4h; | 100% |

-
-
20433-10-7
5-(4-methoxyphenyl)-2-phenyl-2H-tetrazole

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In ethyl acetate for 2h; UV-irradiation; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 0 - 20℃; for 16h; | 100% |
-
-
159665-98-2
Cr(CO)3(C9(CH3)7)Rh(CO)2

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In dichloromethane Ar-atmosphere; 35°C (1 h); evapn. (Ar-stream), washing (pentane); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In acetone byproducts: C3H2N2(CH3)2(C3H7)Br; reductive elimination for 3 h; | 100% |


-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78℃; Diels-Alder reaction; | 100% |

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78℃; Diels-Alder reaction; | 100% |

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78℃; Diels-Alder reaction; | 100% |

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78℃; Diels-Alder reaction; | 100% |
-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78℃; Diels-Alder reaction; | 100% |
-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78℃; Diels-Alder reaction; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 8h; Diels-Alder cycloaddition; | 100% |
-
-
12078-25-0
dicarbonylcyclopentadienylcobalt

-
-
624-49-7
dimethylfumarate

| Conditions | Yield |
|---|---|
| In toluene Irradiation (UV/VIS); reflux, 3 h; flash chromy. on silica gel; | 100% |
| In toluene for 3h; Reflux; Irradiation; |
-
-
189559-57-7
1,2-bis(diphenylphosphino)ethane hydridoplatinum molybdenum cyclopentadiene (CO)3

-
-
624-49-7
dimethylfumarate

-
A
-
12176-06-6
hydrido(tricarbonyl)(cyclopentadienyl)molybdenum

| Conditions | Yield |
|---|---|
| In benzene-d6 at 30℃; for 0.25h; Inert atmosphere; Schlenk technique; | A 100% B 74% |

Dimethyl fumarate Chemical Properties
Molecule structure of Dimethyl fumarate (CAS NO.624-49-7):
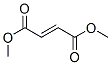
IUPAC Name: Dimethyl (E)-but-2-enedioate
Molecular Weight: 144.12532 g/mol
Molecular Formula: C6H8O4
Density: 1.124 g/cm3
Melting Point: 102-106 °C(lit.)
Boiling Point: 193 °C at 760 mmHg
Flash Point: 91.1 °C
Index of Refraction: 1.435
Molar Refractivity: 33.45 cm3
Molar Volume: 128.1 cm3
Surface Tension: 33.1 dyne/cm
Enthalpy of Vaporization: 42.92 kJ/mol
Vapour Pressure: 0.475 mmHg at 25 °C
Storage Temp. store at RT.
Stability: stable. incompatible with acids, bases, oxidizing agents, reducing agents.
XLogP3: 0.7
H-Bond Acceptor: 4
Rotatable Bond Count: 4
Exact Mass: 144.042259
MonoIsotopic Mass: 144.042259
Topological Polar Surface Area: 52.6
Heavy Atom Count: 10
Canonical SMILES: COC(=O)C=CC(=O)OC
Isomeric SMILES: COC(=O)/C=C/C(=O)OC
InChI: InChI=1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3+
InChIKey: LDCRTTXIJACKKU-ONEGZZNKSA-N
EINECS: 210-849-0
Product Categories: Acids & Esters; C6 to C7; Carbonyl Compounds; Esters
Dimethyl fumarate Uses
Dimethyl fumarate (CAS NO.624-49-7) is widely used in food, feed, tobacco, leather or other anticorrosion ,mouldproof and preservation. Dimethyl fumarate is used to treat psoriasis. It is a lipophilic, highly mobile molecule in human tissue. Another use for dimethyl fumarate is mold inhibition, mostly for leather products.
Dimethyl fumarate Toxicity Data With Reference
| 1. | skn-rbt 20 mg/24H MOD | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,373. | ||
| 2. | eye-rbt 250 µg/24H SEV | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,373. | ||
| 3. | orl-rat LD50:2240 mg/kg | AIHAAP American Industrial Hygiene Association Journal. 30 (1969),470. | ||
| 4. | skn-rbt LD50:1250 mg/kg | AIHAAP American Industrial Hygiene Association Journal. 30 (1969),470. |
Dimethyl fumarate Consensus Reports
Reported in EPA TSCA Inventory.
Dimethyl fumarate Safety Profile
Hazard Codes:  Xn
Xn
Risk Statements: 21-38-41-43-36/37/38
R21:Harmful in contact with skin.
R38:Irritating to skin.
R41:Risk of serious damage to the eyes.
R43:May cause sensitization by skin contact.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 1
RTECS: EM6125000
HS Code: 29171990
Moderately toxic by ingestion and skin contact. A skin and severe eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Dimethyl fumarate Specification
Dimethyl fumarate (CAS NO.624-49-7) is also named as 2-Butenedioic acid, dimethyl ester, (E)- ; 4-02-00-02205 (Beilstein Handbook Reference) ; AI3-07872 ; Allomaleic acid dimethyl ester ; BG 00012 ; BG 12 compound ; BG-00012 ; BG00012 ; BRN 0774590 ; Boletic acid dimethyl ester ; Dimethyl trans-ethylenedicarboxylate ; Dimethylester kyseliny fumarove ; Dimethylester kyseliny fumarove [Czech] ; Dimethylfumarate ; Ethylene, 1,2-bis(methoxycarbonyl)-, trans- ; FAG 201 ; FAG201 ; Fumaderm ; Fumaric acid, dimethyl ester ; Methyl fumarate ; NSC 167432 ; NSC 25942 ; TL 353 ; UNII-FO2303MNI2 ; trans-1,2-Ethylenedicarboxylic acid dimethyl ester ; trans-Butenedioic acid dimethyl ester . Dimethyl fumarate (CAS NO.624-49-7) is fine white crystalline powder.
Related Products
- Dimethyl ( )-2,3-O-Isopropylidene-D-tartrate
- Dimethyl ((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)propanedioate
- Dimethyl (2-oxo-3,3-difluoroheptyl)phosphonate
- Dimethyl (2-oxo-4-phenylbutyl)phosphonate
- Dimethyl (2-oxoheptyl)phosphonate
- Dimethyl (2S, 2'S)-1, 1'-((2S, 2'S)-2, 2'-(4, 4'-(biphenyl-4, 4'-diyl)bis(1H-imidazole-4, 2-diyl))bis(pyrrolidine-2, 1-diyl))bis(3-methyl-1-oxobutane-2, 1-diyl)dicarbamate
- Dimethyl (3-phenoxy-2-oxopropyl)phosphonate
- Dimethyl (R)-(+)-methylsuccinate
- Dimethyl (S)-(-)-methylsuccinate
- Dimethyl (S)-3-hydroxy-L-aspartate
- 6245-04-1
- 624-51-1
- 62451-84-7
- 62451-88-1
- 62452-73-7
- 62455-56-5
- 62455-63-4
- 6245-59-6
- 6245-60-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View