-
Name
Estradiol
- EINECS 200-354-8
- CAS No. 57-91-0
- Article Data39
- CAS DataBase
- Density 1.17 g/cm3
- Solubility ethanol: 50 mg/mL, clear, colorless
- Melting Point 176-180 °C(lit.)
- Formula C18H24O2
- Boiling Point 445.9 °C at 760 mmHg
- Molecular Weight 272.387
- Flash Point 209.6 °C
- Transport Information UN 2811 6.1/PG 2
- Appearance White solid
- Safety 53-45-24/25-22
- Risk Codes 45-48-40
-
Molecular Structure
-
Hazard Symbols
 T,
T,  Xn
Xn
- Synonyms 17a-Estradiol (8CI);1,3,5-Estratriene-3,17a-diol;13b-Methyl-1,3,5(10)-gonatriene-3,17a-diol;17-Epiestradiol;17a-Oestradiol;3,17-Dihydroxyestratriene;3,17a-Dihydroxyestra-1,3,5(10)-triene;3,17a-Dihydroxyoestra-1,3,5(10)-triene;Alfatradiol;Epiestrol;Estra-1,3,5(10)-triene-3,17a-diol;NSC 20293;Oestra-1,3,5(10)-triene-3,17a-diol;a-Estradiol;
- PSA 40.46000
- LogP 3.60920
Synthetic route
-
-
1474-52-8
estradiol-3,17α-diacetate

-
A
-
57-91-0
α-estradiol

-
B
-
15068-99-2
17α-acetoxy-estra-1,3,5(10)-trien-3-ol

| Conditions | Yield |
|---|---|
| With lipase from Candida cylindracea; octanol In various solvent(s) at 37℃; for 144h; | A 60% B 25% |

| Conditions | Yield |
|---|---|
| With propan-1-ol; sodium |


| Conditions | Yield |
|---|---|
| With sodium |

| Conditions | Yield |
|---|---|
| With ethanol; nickel at 120℃; under 14710.2 Torr; Hydrogenation; | |
| With potassium hydroxide; aluminum nickel at 80 - 90℃; | |
| With aluminum isopropoxide; isopropyl alcohol |

| Conditions | Yield |
|---|---|
| Isolierung aus dem Harn von weiblichen Kaninchen nach Injektion von (+)-Oestron; | |
| Multi-step reaction with 2 steps 1: p-toluenesulfonic acid / methanol 2: NaBH4 / propan-2-ol; H2O / 16 h / Ambient temperature View Scheme |
-
-
53-16-7
Estrone

-
-
555-31-7
aluminum isopropoxide

-
-
67-63-0
isopropyl alcohol

-
A
-
50-28-2
estradiol

-
B
-
57-91-0
α-estradiol

-
-
2393-53-5
estrone 3-benzoate

-
-
555-31-7
aluminum isopropoxide

-
-
67-63-0
isopropyl alcohol

-
A
-
50-28-2
estradiol

-
B
-
57-91-0
α-estradiol

-
-
50-28-2
estradiol

-
A
-
53-16-7
Estrone

-
B
-
481-97-0
estrone sulfate

-
C
-
57-91-0
α-estradiol

-
D
-
481-96-9
estradiol-3-sulfate

-
E
-
22139-70-4
17-alpha-estradiol-3-sulfate

-
F
-
3233-69-0, 47328-45-0
estradiol-17ξ 17-sulfate

| Conditions | Yield |
|---|---|
| Product distribution; Mechanism; metabolism after i. v. injection in White Leghorn laying hens, <3H>labelled; |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water; isopropyl alcohol for 16h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| at 80 - 90℃; |


-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With potassium acetate; N,N-dimethyl-formamide anschliessendes Behandeln mit methanol. KOH; |

-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride |

-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With potassium acetate; N,N-dimethyl-formamide anschliessendes Behandeln mit methanol. KOH; |
-
-
64-17-5
ethanol

-
-
6639-99-2
17α-dihydroequilenin

-
A
-
57-91-0
α-estradiol

-
B
-
517-08-8
estratriene-(B)-diol-(3α.17α)

-
C
-
517-07-7, 517-08-8, 23356-38-9, 23356-39-0, 50395-28-3, 100163-40-4, 104874-68-2
estratriene-(B)-diol-(3β.17α)

-
-
71-41-0
pentan-1-ol

-
-
6639-99-2
17α-dihydroequilenin

-
A
-
57-91-0
α-estradiol

-
B
-
517-08-8
estratriene-(B)-diol-(3α.17α)

-
C
-
517-07-7, 517-08-8, 23356-38-9, 23356-39-0, 50395-28-3, 100163-40-4, 104874-68-2
estratriene-(B)-diol-(3β.17α)


| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In toluene |

| Conditions | Yield |
|---|---|
| With tris(2-carboxyethyl)phosphine In water; acetonitrile pH=7.4; aq. phosphate buffer; Inert atmosphere; |

-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water for 0.5h; Reflux; | 378.4 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; tetra(n-butyl)ammonium hydrogensulfate / dichloromethane; water 1.2: 2 h / 22 °C 2.1: di-isopropyl azodicarboxylate; triphenylphosphine / toluene / 1 h / 25 - 100 °C 3.1: sodium hydroxide / ethanol; water / 0.5 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 2-(diphenylphosphino)pyridine / toluene / 10 h / Cooling with ice 1.2: 20 h / 80 °C 2.1: copper(ll) bromide; lithium bromide / ethyl acetate / 4.5 h / 90 °C 3.1: potassium hydroxide / water; ethanol / 16 h / 95 °C View Scheme |

-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water at 95℃; for 16h; |
-
-
605-65-2
5-(dimethylamino)naphth-1-ylsulfonyl chloride

-
-
57-91-0
α-estradiol

-
-
100821-85-0
DNS-α-estradiol

| Conditions | Yield |
|---|---|
| Duolite A-102D In acetone for 0.0833333h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: α-estradiol; sodium hydride In tetrahydrofuran at 22℃; for 1h; Stage #2: With triethylamine sulfur trioxide In tetrahydrofuran at 22℃; Product distribution / selectivity; | 90% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; potassium carbonate In acetonitrile for 48h; Reflux; | 89% |

| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; for 1h; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: α-estradiol; sodium methylate In tetrahydrofuran; methanol at 22℃; for 0.5h; Stage #2: With sulfur trioxide trimethylamine complex In tetrahydrofuran; methanol at 22℃; for 20h; Product distribution / selectivity; | 86% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; water; potassium carbonate In N,N-dimethyl-formamide at 60℃; for 8h; Substitution; | 85% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 72h; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; potassium carbonate In acetonitrile for 72h; Reflux; | 80% |

-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium tert-butylate In N,N-dimethyl-formamide stirring Fe-complex, steroid and t-BuOK, addn. of excess 10% HCl and then NH4PF6; | 77% |
| With hydrogenchloride; potassium carbonate In N,N-dimethyl-formamide stirring Fe-complex, steroid and K2CO3 at 55°C for 4-6 h, coolingto room temp., addn. of excess 10% HCl and then NH4PF6; partial evapn. (reduced pressure), extn. into CH2Cl2 (or CH2Cl2/MeNO2=1:1), drying (Na2SO4), evapn., chromy. (Al2O3, CCl4 or CHCl3; then CH2Cl2/CHCl3), evapn., drying (vac. desiccator); elem. anal.; | 77% |
| With hydrogenchloride; potassium carbonate In tetrahydrofuran; dimethyl sulfoxide stirring Fe-complex, steroid and K2CO3 in THF/DMSO=9:1 at 55°C for 4-6 h, cooling to room temp., addn. of excess 10% HCl and then NH4PF6; partial evapn. (reduced pressure), extn. into CH2Cl2 (or CH2Cl2/MeNO2=1:1), drying (Na2SO4), evapn., chromy. (Al2O3, CCl4 or CHCl3; then CH2Cl2/CHCl3), evapn., drying (vac. desiccator); |

-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With Amberlyst A26-resin bound iodine azide In acetonitrile at 20℃; for 2h; Catalytic behavior; Inert atmosphere; | 76% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water at 65℃; for 0.5h; | 75% |
| With sodium hydroxide |
-
-
57-91-0
α-estradiol

| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.333333h; Ambient temperature; | 72% |
-
-
50-00-0
formaldehyd

-
-
57-91-0
α-estradiol

-
A
-
1336906-93-4
(8R,9S,13S,14S,17R)-3,17-dihydroxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-4-carbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; α-estradiol With triethylamine; magnesium chloride In tetrahydrofuran Inert atmosphere; Reflux; Stage #2: With lithium hydroxide In methanol for 0.25h; Stage #3: With hydrogenchloride In methanol; water | A n/a B 69% |


| Conditions | Yield |
|---|---|
| In water; acetone for 24h; pH=7; Time; Microbiological reaction; regioselective reaction; | 69% |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane at 20℃; for 3h; Inert atmosphere; | 39.6% |

| Conditions | Yield |
|---|---|
| With sulphamoyl chloride In acetonitrile for 10h; | A 14.6% B 35.4% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide | |
| Alkaline conditions; |

| Conditions | Yield |
|---|---|
| With pyridine; dichloromethane |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In chloroform |
Epiestradiol Specification
The Epiestradiol is an organic compound with the formula C18H24O2. The IUPAC name of this chemical is (8R,9S,13S,14S,17R)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol. With the CAS registry number 57-91-0, it is also named as 17-Epiestradiol. The product's category is Steroids. It is used in form of an ethanolic solution for topical application on the scalp. Similarly to other drugs against alopecia, topical or oral, it has to be applied continuously to prevent further hair loss.
Physical properties about Epiestradiol are: (1)ACD/LogP: 4.13; (2)ACD/LogD (pH 5.5): 4.13; (3)ACD/LogD (pH 7.4): 4.13; (4)ACD/BCF (pH 5.5): 811.75; (5)ACD/BCF (pH 7.4): 810.7; (6)ACD/KOC (pH 5.5): 4208.86; (7)ACD/KOC (pH 7.4): 4203.38; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 2; (11)Polar Surface Area: 18.46 Å2; (12)Index of Refraction: 1.599; (13)Molar Refractivity: 79.5 cm3; (14)Molar Volume: 232.6 cm3; (15)Polarizability: 31.51×10-24cm3; (16)Surface Tension: 48.9 dyne/cm; (17)Density: 1.17 g/cm3; (18)Flash Point: 209.6 °C; (19)Enthalpy of Vaporization: 74.19 kJ/mol; (20)Boiling Point: 445.9 °C at 760 mmHg; (21)Vapour Pressure: 9.82E-09 mmHg at 25°C.
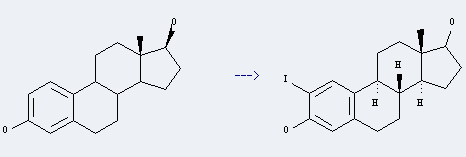
Uses of Epiestradiol: it can be used to produce 2-iodoestradiol at temperature of 60 °C. It will need reagent iodine, copper(II) acηte didydrate and solvent acetic acid with reaction time of 22 hours. The yield is about 64%.
When you are using this chemical, please be cautious about it as the following:
It may cause cancer and danger of serious damage to health by prolonged exposure. Please avoid exposure - obtain special instructions before use. Besides, this chemical has limited evidence of a carcinogenic effect. When you are using it, do not breathe dust and avoid contact with skin and eyes. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: Oc1cc3c(cc1)[C@H]2CC[C@@]4([C@H](O)CC[C@H]4[C@@H]2CC3)C
(2)InChI: InChI=1/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17-,18+/m1/s1
(3)InChIKey: VOXZDWNPVJITMN-SFFUCWETBJ
(4)Std. InChI: InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17-,18+/m1/s1
(5)Std. InChIKey: VOXZDWNPVJITMN-SFFUCWETSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View