-
Name
3-ACETYLPHENANTHRENE
- EINECS 218-020-5
- CAS No. 2039-76-1
- Article Data11
- CAS DataBase
- Density 1.164 g/cm3
- Solubility
- Melting Point 67-71 °C(lit.)
- Formula C16H12O
- Boiling Point 405.9 °C at 760 mmHg
- Molecular Weight 220.271
- Flash Point 180.7 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ketone,methyl 3-phenanthryl (6CI,7CI,8CI);1-(3-Phenanthryl)ethanone;3-Acetylphenanthrene;Methyl 3-phenanthryl ketone;NSC 3192;1-(3-phenanthryl)ethan-1-one;1-(3-Phenanthryl)ethanone;Ethanone, 1- (3-phenanthrenyl)-;ethanone, 1-(3-phenanthrenyl)-;Ketone, methyl 3-phenanthryl;
- PSA 17.07000
- LogP 4.19560
Synthetic route

-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| palladium on activated charcoal at 300℃; for 4.5h; | 73% |

| Conditions | Yield |
|---|---|
| With potassium iodide In cyclohexane for 3.75h; Irradiation; | 43% |
-
-
1261648-81-0
1-(5-bromo-2-iodophenyl)ethanone

-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; potassium carbonate; palladium dichloride In N,N-dimethyl-formamide at 150℃; for 3h; Inert atmosphere; Sealed tube; | 30% |
-
-
85-01-8
phenanthrene

-
-
108-24-7
acetic anhydride

-
A
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
B
-
5968-48-9
1-Phenanthren-1-yl-ethanone

| Conditions | Yield |
|---|---|
| With hydrogen fluoride at 50 - 60℃; |

-
-
85-01-8
phenanthrene

-
-
75-36-5
acetyl chloride

-
A
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
B
-
5968-48-9
1-Phenanthren-1-yl-ethanone

| Conditions | Yield |
|---|---|
| With aluminium trichloride; nitrobenzene |


| Conditions | Yield |
|---|---|
| With aluminium trichloride |
-
-
85-01-8
phenanthrene

-
-
75-36-5
acetyl chloride

-
A
-
2039-77-2
9-acetylphenanthrene

-
B
-
5960-69-0
2-acetylphenanthrene

-
C
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| With aluminium trichloride for 30h; Ambient temperature; Further byproducts given; | A 70.6 % Chromat. B 4.0 % Chromat. C 25.0 % Chromat. |
| With aluminium trichloride Product distribution; Ambient temperature; various time and molar ratio AlCl3/1a; | A 26.5 % Chromat. B 17.1 % Chromat. C 55.1 % Chromat. |
| With aluminium trichloride for 52h; Ambient temperature; | A 26.5 % Chromat. B 17.1 % Chromat. C 55.1 % Chromat. |

-
-
85-01-8
phenanthrene

-
-
7664-39-3
hydrogen fluoride

-
-
108-24-7
acetic anhydride

-
A
-
5960-69-0
2-acetylphenanthrene

-
B
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| at 50 - 55℃; |

-
-
75-15-0
carbon disulfide

-
-
7446-70-0
aluminium trichloride

-
-
85-01-8
phenanthrene

-
-
75-36-5
acetyl chloride

-
A
-
5960-69-0
2-acetylphenanthrene

-
B
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| hoehere Temperaturen; |

-
-
7446-70-0
aluminium trichloride

-
-
85-01-8
phenanthrene

-
-
98-95-3
nitrobenzene

-
-
75-36-5
acetyl chloride

-
A
-
5960-69-0
2-acetylphenanthrene

-
B
-
2039-76-1
1-(phenanthren-3-yl)ethanone


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 45 percent / H2SO4 / 6.5 h / Ambient temperature 2: 73 percent / 10percent Pd/C / 4.5 h / 300 °C View Scheme |

-
-
85-01-8
phenanthrene

-
-
75-36-5
acetyl chloride

-
A
-
5960-69-0
2-acetylphenanthrene

-
B
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In nitrobenzene Friedel Crafts acylation; |

-
-
29124-56-9
1-(2-amino-5-bromophenyl)ethanone

-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: toluene-4-sulfonic acid; potassium iodide; sodium nitrite / acetonitrile; water / 4.5 h / 10 - 20 °C / Inert atmosphere; Sealed tube 2: palladium dichloride; 1,3-bis-(diphenylphosphino)propane; potassium carbonate / N,N-dimethyl-formamide / 3 h / 150 °C / Inert atmosphere; Sealed tube View Scheme |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
34585-56-3
2-bromo-3'-acetophenanthrone

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In chloroform; ethyl acetate for 6h; Reflux; | 100% |
| With bromine In 1,4-dioxane; diethyl ether at 20℃; for 3h; | 56% |
| With copper(ll) bromide In chloroform; ethyl acetate for 3.5h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium hypochlorite In 1,4-dioxane at 65℃; for 3h; | 100% |
| With sodium hypochlorite; sodium hydroxide In 1,4-dioxane at 65℃; for 6h; | 97% |
| With sodium hypochlorite solution In 1,4-dioxane; water at 120℃; for 24h; | 92% |

| Conditions | Yield |
|---|---|
| With selenium (IV) oxide In 1,4-dioxane; water Heating; | 99% |
| With selenium(IV) oxide In toluene | |
| With selenium(IV) oxide |

| Conditions | Yield |
|---|---|
| In methanol at 60℃; | 98% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 12h; Ambient temperature; | 97% |
| With ethanol; platinum Hydrogenation; | |
| With copper oxide-chromium oxide; barium(II) oxide; decalin at 100℃; under 97822.5 Torr; Hydrogenation; | |
| With aluminum isopropoxide; isopropyl alcohol | |
| With lithium aluminium tetrahydride; diethyl ether |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
100-52-7
benzaldehyde

-
-
62953-48-4
1-[3]phenanthryl-3-phenyl-propenone

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.0666667h; Microwave irradiation; | 96% |
| With sodium hydroxide In ethanol for 48h; Ambient temperature; | 68.1% |

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.0666667h; Microwave irradiation; | 95% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
620-23-5
m-tolyl aldehyde

-
-
63681-63-0
(Z)-1-Phenanthren-3-yl-3-m-tolyl-propenone

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.0666667h; Microwave irradiation; | 95% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
555-16-8
4-nitrobenzaldehdye

-
-
31907-28-5
3-(4-nitro-phenyl)-1-[3]phenanthryl-propenone

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.1h; Microwave irradiation; | 94% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
38374-12-8
(E)-3-(4-Bromo-phenyl)-1-phenanthren-3-yl-propenone

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.075h; Microwave irradiation; | 93% |

| Conditions | Yield |
|---|---|
| With 4-nitrophenyl azide; acetic acid In toluene at 100℃; for 12h; Molecular sieve; | 91% |

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.075h; Microwave irradiation; | 91% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
552-89-6
2-nitro-benzaldehyde

-
-
63646-43-5
(Z)-3-(2-Nitro-phenyl)-1-phenanthren-3-yl-propenone

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.1h; Microwave irradiation; | 91% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
89-98-5
2-chloro-benzaldehyde

-
-
31919-49-0
(E)-3-(2-Chloro-phenyl)-1-phenanthren-3-yl-propenone

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.0666667h; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.0666667h; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With FeCl3-bentonite for 0.0833333h; Microwave irradiation; | 90% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| Stage #1: 1-(phenanthren-3-yl)ethanone With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: 1,5-bis-(morpholino)-pentadienylium triflate In tetrahydrofuran; hexane at -78 - 20℃; for 3.25h; Further stages.; | 86% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ammonium nitrate; iodine; oxygen In water; acetonitrile at 60℃; for 22h; Green chemistry; chemoselective reaction; | 85% |
-
-
110-91-8
morpholine

-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
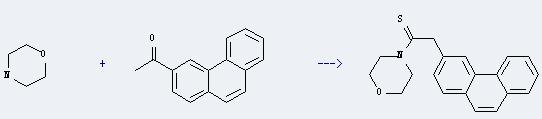
117752-99-5
3-Phenanthrylacetothiomorpholide

| Conditions | Yield |
|---|---|
| With sulfur for 16h; Heating; | 82% |
| With sulfur |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
3490-92-4
methyl 2-cyano-3,3-bis(methylsulfanyl)acrylate

-
-
681449-07-0
4-methylsulfanyl-2-oxo-6-(phenanthren-3-yl)-2H-pyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 10h; | 82% |

| Conditions | Yield |
|---|---|
| With pyridine; hydroxylamine hydrochloride In ethanol at 75℃; for 1h; | 81% |
| Multi-step reaction with 2 steps 1.1: pyridine; hydroxylamine hydrochloride / ethanol / 3 h / Heating / reflux 2.1: PPA / 2.5 h / 100 °C 2.2: Heating / reflux View Scheme |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
343338-28-3
(S)-2-methylpropane-2-sulfinamide

| Conditions | Yield |
|---|---|
| With titanium(IV) tetraethanolate In tetrahydrofuran for 96h; Inert atmosphere; Reflux; | 78% |

| Conditions | Yield |
|---|---|
| With pyridine; hydroxylamine hydrochloride In ethanol at 100℃; for 2h; Inert atmosphere; | 75.1% |
| With pyridine; hydroxylamine hydrochloride In ethanol for 3h; Heating / reflux; | |
| With pyridine; hydroxylamine hydrochloride In ethanol for 3h; Heating / reflux; |

| Conditions | Yield |
|---|---|
| With iodine(I) bromide In chlorobenzene at 130℃; for 4h; Sealed tube; | 75% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
228266-66-8
3-(1-chloroethenyl)phenanthrene

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride; phosphorus trichloride for 24h; Ambient temperature; | 69% |
-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 2.25h; Ambient temperature; Title compound not separated from byproducts; | A 25% B 66% |


| Conditions | Yield |
|---|---|
| With selenium; oxygen; iodine(I) bromide In 1-methyl-pyrrolidin-2-one at 140℃; Sealed tube; | 63% |
| With selenium; [bis(acetoxy)iodo]benzene; Iodine monochloride In 1-methyl-pyrrolidin-2-one at 140℃; for 18h; | 29% |
-
-
50-00-0
formaldehyd

-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

-
-
10024-89-2
morpholin hydrochloride

-
-
7470-64-6
3-morpholino-1-(phenanthren-3-yl)propan-1-one hydrochloride

| Conditions | Yield |
|---|---|
| In i-Amyl alcohol Reflux; | 60% |
| Mannich reaction; |

-
-
2039-76-1
1-(phenanthren-3-yl)ethanone

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In ethanol Heating; | 59% |
| With pyridine; hydroxylamine hydrochloride In ethanol for 3h; Substitution; Heating; |
Ethanone,1-(3-phenanthrenyl)- Specification
The Ethanone,1-(3-phenanthrenyl)-, with the CAS registry number 2039-76-1 and EINECS registry number 218-020-5, has the IUPAC name of 1-phenanthren-3-ylethanone. It belongs to the following product categories: API intermediates; C15 to C38; Carbonyl Compounds; Ketones. And the molecular formula of the chemical is C16H12O.
The physical properties of Ethanone,1-(3-phenanthrenyl)- are as followings: (1)ACD/LogP: 4.13; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 4.13; (4)ACD/LogD (pH 7.4): 4.13; (5)ACD/BCF (pH 5.5): 805.41; (6)ACD/BCF (pH 7.4): 805.41; (7)ACD/KOC (pH 5.5): 4185.3; (8)ACD/KOC (pH 7.4): 4185.3; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.685; (14)Molar Refractivity: 71.96 cm3; (15)Molar Volume: 189.1 cm3; (16)Polarizability: 28.52×10-24cm3; (17)Surface Tension: 48.8 dyne/cm; (18)Density: 1.164 g/cm3; (19)Flash Point: 180.7 °C; (20)Enthalpy of Vaporization: 65.75 kJ/mol; (21)Boiling Point: 405.9 °C at 760 mmHg; (22)Vapour Pressure: 8.47E-07 mmHg at 25°C.
Uses of Ethanone,1-(3-phenanthrenyl)-: It can react with morpholine to produce 4-[3]phenanthrylsulfanylacetyl-morpholine. This reaction will need reagent S. The reaction time is 16 hours with heating, and the yield is about 82%.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(c3ccc2ccc1ccccc1c2c3)C
(2)InChI: InChI=1/C16H12O/c1-11(17)14-9-8-13-7-6-12-4-2-3-5-15(12)16(13)10-14/h2-10H,1H3
(3)InChIKey: JKVNPRNAHRHQDD-UHFFFAOYAK
Related Products
- Ethanone, 1-(1,2,3,4-tetrahydro-6-quinolinyl)-
- Ethanone, 1-(1-chlorocyclopropyl)-
- Ethanone, 1-(1-methyl-1H-imidazol-5-yl)-
- Ethanone, 1-(1-methyl-1H-pyrazol-5-yl)- (9CI)
- Ethanone, 1-(2,3-difluoro-4-methylphenyl)-
- Ethanone, 1-(2,4-dimethyl-5-oxazolyl)-
- Ethanone, 1-(2-amino-5-methoxyphenyl)-
- Ethanone, 1-(2-ethoxyphenyl)-
- Ethanone, 1-(2-phenoxyphenyl)-
- Ethanone, 1-(3,4-dihydro-2H-1,4-benzoxazin-6-yl)-
- 2039-80-7
- 2039-82-9
- 203983-14-6
- 20398-34-9
- 2039-85-2
- 2039-86-3
- 2039-87-4
- 2039-88-5
- 2039-89-6
- 2039-96-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View