-
Name
Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate
- EINECS 1806241-263-5
- CAS No. 74892-82-3
- Article Data6
- CAS DataBase
- Density 0.969 g/cm3
- Solubility
- Melting Point
- Formula C9H17NO2
- Boiling Point 226.1 °C at 760 mmHg
- Molecular Weight 171.239
- Flash Point 90.5 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Piperidinecarboxylicacid, 4-methyl-, ethyl ester, (2R-trans)-;(2R,4R)-4-Methyl-2-piperidinecarboxylicacid ethyl ester;2-Piperidinecarboxylicacid, 4-methyl-, ethyl ester, (2R,4R)-;
- PSA 38.33000
- LogP 1.26640
Synthetic route
-
-
131278-84-7
ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate L-(+)-tartarate

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; ethyl acetate at 15 - 20℃; for 1h; | 88% |
-
-
198641-56-4
ethyl (2R,4R)-4-methyl-1-((S)-1-phenylethyl)tetrahydropyridine-2-carboxylate

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With 10% palladium hydroxide on charcoal; hydrogen In ethanol at 20℃; under 760.051 Torr; for 4h; | 45% |
| With palladium 10% on activated carbon; hydrogen; iron(II) oxalate; acetic acid In ethanol at 30℃; under 3750.38 Torr; for 4h; Reagent/catalyst; Autoclave; | 345 g |
-
-
134984-62-6, 134984-77-3
(2R,4R)-4-Methyl-1-((R)-1-phenyl-ethyl)-piperidine-2-carboxylic acid ethyl ester

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogen; palladium hydroxide - carbon In ethanol under 760 Torr; Ambient temperature; |
-
-
139334-62-6
(6R)-1-<(R)-1-phenylethyl>-6-ethoxycarbonyl-4-methyl-3,4-didehydropiperidine

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / 3percent Pt-C / ethyl acetate / 16 h / 760 Torr / Ambient temperature 2: H2 / Pd(OH)2-C / ethanol / 760 Torr / Ambient temperature View Scheme |
-
-
78-79-5
isoprene

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: TFA, H2O / dimethylformamide / 30 h / Ambient temperature 2: H2 / 3percent Pt-C / ethyl acetate / 16 h / 760 Torr / Ambient temperature 3: H2 / Pd(OH)2-C / ethanol / 760 Torr / Ambient temperature View Scheme |
-
-
139334-63-7
ethyl (2R)-4-methyl-1-((S)-1-phenylethyl)-1,2,3,6-tetrahydropyridine-2-carboxylate

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (SP, S′P)-1,1′-bis[bis(4-methoxy-3,5-dimethylphenyl)phosphino]-2,2′-bis[(R)-α-(dimethylamino)benzyl]ferrocene; bis(norbornadiene)rhodium(l)tetrafluoroborate; methanesulfonic acid; hydrogen / ethanol / 18 h / 90 °C / 7500.75 Torr / Autoclave 2: 10% palladium hydroxide on charcoal; hydrogen / ethanol / 4 h / 20 °C / 760.05 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: 3 % platinum on carbon; hydrogen / ethyl acetate / 48 h / 20 °C / 760.05 Torr 2: 10% palladium hydroxide on charcoal; hydrogen / ethanol / 4 h / 20 °C / 760.05 Torr View Scheme |

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; ethyl acetate at 20℃; for 1h; |
-
-
626-58-4
4-methylpiperidin

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: acetic acid; sodium hypochlorite / water / 2 h / 0 - 10 °C 1.2: 0 - 20 °C 2.1: hydrogenchloride / water; dichloromethane / 1.5 h / 0 - 10 °C 3.1: water; dichloromethane / 0 - 20 °C 4.1: hydrogenchloride / water / 20 - 60 °C 5.1: thionyl chloride / 0 °C / Reflux 6.1: ethanol; acetone / 0.5 h / 40 °C / Resolution of racemate 7.1: potassium carbonate / water; ethyl acetate / 1 h / 15 - 20 °C View Scheme |
-
-
85796-64-1
2,3,4,5-tetrahydro-4-methylpyridine

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: hydrogenchloride / water; dichloromethane / 1.5 h / 0 - 10 °C 2: water; dichloromethane / 0 - 20 °C 3: hydrogenchloride / water / 20 - 60 °C 4: thionyl chloride / 0 °C / Reflux 5: ethanol; acetone / 0.5 h / 40 °C / Resolution of racemate 6: potassium carbonate / water; ethyl acetate / 1 h / 15 - 20 °C View Scheme |

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: water; dichloromethane / 0 - 20 °C 2: hydrogenchloride / water / 20 - 60 °C 3: thionyl chloride / 0 °C / Reflux 4: ethanol; acetone / 0.5 h / 40 °C / Resolution of racemate 5: potassium carbonate / water; ethyl acetate / 1 h / 15 - 20 °C View Scheme |

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogenchloride / water / 20 - 60 °C 2: thionyl chloride / 0 °C / Reflux 3: ethanol; acetone / 0.5 h / 40 °C / Resolution of racemate 4: potassium carbonate / water; ethyl acetate / 1 h / 15 - 20 °C View Scheme |

-
A
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
B
-
78306-52-2
ethyl (2S,4S)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; ethyl acetate at 15 - 20℃; for 1.08333h; Overall yield = 85 %; Overall yield = 29.4 g; | A n/a B n/a |

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol; acetone / 0.5 h / 40 °C / Resolution of racemate 2: potassium carbonate / water; ethyl acetate / 1 h / 15 - 20 °C View Scheme |

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: thionyl chloride / 0 °C / Reflux 2: ethanol; acetone / 0.5 h / 40 °C / Resolution of racemate 3: potassium carbonate / water; ethyl acetate / 1 h / 15 - 20 °C View Scheme |
-
-
153886-63-6
1,2,3,4-tetrahydro-3-methyl-8-quinolinesulfonyl chloride

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: N-Boc-N'-nitro-L-arginine; ethyl (2R-trans)-4-methylpiperidine-2-carboxylate With 4-methyl-morpholine; 2-chloro-4,6-dimethoxy-1 ,3,5-triazine In chloroform at -30℃; for 2h; Stage #2: 1,2,3,4-tetrahydro-3-methyl-8-quinolinesulfonyl chloride In chloroform at 20℃; for 3h; Temperature; | 87.5% |

-
-
2480-93-5
Boc-Orn(Z)-OH

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
174699-01-5
(2R,4R)-1-((S)-5-Benzyloxycarbonylamino-2-tert-butoxycarbonylamino-pentanoyl)-4-methyl-piperidine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.75h; Acylation; | 86% |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
183679-12-1
5-benzyloxycarbonylamino-2-tert-butoxycarbonylamino-4,4-difluoro-pentanoic acid

-
-
183679-13-2
(2R,4R)-1-((S)-5-Benzyloxycarbonylamino-2-tert-butoxycarbonylamino-4,4-difluoro-pentanoyl)-4-methyl-piperidine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; Acylation; | 84% |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
183679-09-6
(S)-4-(1,3-Dioxo-1,3-dihydro-isoindol-2-yloxy)-2-(3-methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonylamino)-butyric acid

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; Acylation; | 79% |

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: N2-(3-methyl-8-quinolinesulfonyl)-NG-nitro-L-arginine With trichlorophosphate In tetrahydrofuran at -30℃; for 2h; Reflux; Stage #2: ethyl (2R-trans)-4-methylpiperidine-2-carboxylate In tetrahydrofuran for 2h; | 72.6% |
-
-
1676-90-0
Boc-Asp(OtBu)-OH

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethylmorpholine;; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate In N,N-dimethyl-formamide |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
174699-02-6
(2R,4R)-1-((S)-2-Amino-5-benzyloxycarbonylamino-pentanoyl)-4-methyl-piperidine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86 percent / 1-ethyl-3-(3-dimethylamino)propyl carbodiimide hydrochloride; HOBt; 4-methylmorpholine / dimethylformamide / 0.75 h / 20 °C 2: 100 percent / TFA / 1 h / 0 - 20 °C View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 86 percent / 1-ethyl-3-(3-dimethylamino)propyl carbodiimide hydrochloride; HOBt; 4-methylmorpholine / dimethylformamide / 0.75 h / 20 °C 2: 100 percent / TFA / 1 h / 0 - 20 °C 3: 75 percent / Et3N / CH2Cl2 / 2.75 h / 20 °C 4: 92 percent / H2 / Pd black / ethanol / 72 h / 760 Torr View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 86 percent / 1-ethyl-3-(3-dimethylamino)propyl carbodiimide hydrochloride; HOBt; 4-methylmorpholine / dimethylformamide / 0.75 h / 20 °C 2: 100 percent / TFA / 1 h / 0 - 20 °C 3: 75 percent / Et3N / CH2Cl2 / 2.75 h / 20 °C 4: 92 percent / H2 / Pd black / ethanol / 72 h / 760 Torr 5: 100 percent / NH4OAc / methanol / 24 h / 20 °C View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 86 percent / 1-ethyl-3-(3-dimethylamino)propyl carbodiimide hydrochloride; HOBt; 4-methylmorpholine / dimethylformamide / 0.75 h / 20 °C 2: 100 percent / TFA / 1 h / 0 - 20 °C 3: 75 percent / Et3N / CH2Cl2 / 2.75 h / 20 °C 4: 92 percent / H2 / Pd black / ethanol / 72 h / 760 Torr 5: 100 percent / NH4OAc / methanol / 24 h / 20 °C 6: 40 percent / H2NOH / methanol / 2 h / 20 °C View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
174699-04-8
(2R,4R)-1-[(S)-5-Benzyloxycarbonylamino-2-(3-methyl-quinoline-8-sulfonylamino)-pentanoyl]-4-methyl-piperidine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 86 percent / 1-ethyl-3-(3-dimethylamino)propyl carbodiimide hydrochloride; HOBt; 4-methylmorpholine / dimethylformamide / 0.75 h / 20 °C 2: 100 percent / TFA / 1 h / 0 - 20 °C 3: 75 percent / Et3N / CH2Cl2 / 2.75 h / 20 °C View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 4-ethylmorpholine, (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dimethylformamide 2: p-toluenesulfonic acid monohydrate / acetonitrile / 8 h / Ambient temperature View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
1027984-73-1
(2R,4R)-1-[(S)-3-Carboxy-2-(naphthalene-2-sulfonylamino)-propionyl]-4-methyl-piperidine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 4-ethylmorpholine, (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dimethylformamide 2: p-toluenesulfonic acid monohydrate / acetonitrile / 8 h / Ambient temperature 3: aq. NaHCO3 / dioxane 4: HCl / ethyl acetate / 5 h / Ambient temperature View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
1027037-83-7
(2R,4R)-1-[(S)-3-tert-Butoxycarbonyl-2-(naphthalene-2-sulfonylamino)-propionyl]-4-methyl-piperidine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 4-ethylmorpholine, (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dimethylformamide 2: p-toluenesulfonic acid monohydrate / acetonitrile / 8 h / Ambient temperature 3: aq. NaHCO3 / dioxane View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 4-ethylmorpholine, (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dimethylformamide 2: p-toluenesulfonic acid monohydrate / acetonitrile / 8 h / Ambient temperature 3: aq. NaHCO3 / dioxane 4: HCl / ethyl acetate / 5 h / Ambient temperature 5: 4-ethylmorpholine, (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dimethylformamide View Scheme |
-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
74874-07-0
ethyl (2R,4R)-1-[(2S)-2-[[(1,1-dimethyletoxy)carbonyl]amino]-5-[[imino(nitroamino)methyl]amino]-1-oxopentyl]-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl (2R-trans)-4-methylpiperidine-2-carboxylate With 4-methyl-morpholine; 2-chloro-4,6-dimethoxy-1 ,3,5-triazine In ethyl acetate at -10℃; for 1.5h; Stage #2: ethyl (2R-trans)-4-methylpiperidine-2-carboxylate In ethyl acetate at -10 - 10℃; |
-
-
2188-18-3
Boc-Arg(NO2)-OH

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

-
-
74874-07-0
ethyl (2R,4R)-1-[(2S)-2-[[(1,1-dimethylethoxy)carbonyl]amino]-5-[[imino(nitroamino)methyl]amino]-1-oxopentyl]-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: Boc-Arg(NO2)-OH With 4-methyl-morpholine; 2-chloro-4,6-dimethoxy-1 ,3,5-triazine In ethyl acetate at -10 - -5℃; for 1.5h; Stage #2: ethyl (2R-trans)-4-methylpiperidine-2-carboxylate In ethyl acetate at -5 - 10℃; | 85 g |
-
-
2188-18-3
Boc-Arg(NO2)-OH

-
-
74892-82-3
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 2-chloro-4,6-dimethoxy-1 ,3,5-triazine; 4-methyl-morpholine / ethyl acetate / 1.5 h / -10 - -5 °C 1.2: -5 - 10 °C 2.1: hydrogenchloride / ethanol / 15 - 20 °C View Scheme |
Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate Chemical Properties
Molecule structure of Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate (CAS NO.74892-82-3):
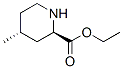
Molecular Weight: 171.24 g/mol
Molecular Formula: C9H17NO2
Density: 0.969 g/cm3
Boiling Point: 226.1 °C at 760 mmHg
Flash Point: 90.5 °C
Index of Refraction: 1.441
Molar Refractivity: 46.69 cm3
Molar Volume: 176.5 cm3
Surface Tension: 29.2 dyne/cm
Enthalpy of Vaporization: 46.26 kJ/mol
Vapour Pressure: 0.0835 mmHg at 25 °C
InChI: InChI=1/C9H17NO2/c1-3-12-9(11)8-6-7(2)4-5-10-8/h7-8,10H,3-6H2,1-2H3/t7-,8-/m1/s1
InChIKey of Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate (CAS NO.74892-82-3): GHBNOCBWSUHAAA-HTQZYQBOBF
Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate Specification
Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate (CAS NO.74892-82-3) is also named as 2-piperidinecarboxylic acid, 4-methyl-, ethyl ester, (2R,4R)- ; Ethyl (2R,4R)-4-methylpiperidine-2-carboxylate ; (2R,4R)-4-Methyl-2-piperidinecarboxylate .
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 74-89-5
- 74896-24-5
- 74896-66-5
- 74899-71-1
- 74901-29-4
- 74901-69-2
- 74-90-8
- 7491-02-3
- 7491-14-7
- 74915-66-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View