-
Name
Ethyl L-alaninate hydrochloride
- EINECS 214-225-9
- CAS No. 1115-59-9
- Article Data32
- CAS DataBase
- Density
- Solubility
- Melting Point 62 °C
- Formula C5H11NO2.HCl
- Boiling Point 127.8 °C at 760 mmHg
- Molecular Weight 153.609
- Flash Point 3.5 °C
- Transport Information
- Appearance White to off-white adhering crystalline powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Alanine,ethyl ester, hydrochloride, L- (6CI,7CI,8CI);L-Alanine, ethyl ester,hydrochloride (9CI);(S)-2-Aminopropionic acid ethyl ester hydrochloride;(S)-Alanine ethyl ester hydrochloride;Ethyl (S)-alaninate hydrochloride;EthylL-alaninate hydrochloride;H-Ala-OEt·HCl;
- PSA 52.32000
- LogP 1.39900
Synthetic route

| Conditions | Yield |
|---|---|
| With thionyl chloride for 4h; Reflux; | 100% |
| With hydrogenchloride for 12h; Reflux; | 99% |
| With thionyl chloride | 99% |

| Conditions | Yield |
|---|---|
| In ethanol | 98% |

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium tetrahydroborate In tetrahydrofuran; water at 20℃; for 2h; Inert atmosphere; Green chemistry; chemoselective reaction; | 91% |
-
-
51814-53-0
N-tert-butoxycarbonyl-L-alanine ethyl ester

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In 1,4-dioxane |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
189060-51-3
(3R,5S)-7-chloro-5-(2,3-dimethoxyphenyl)-1-(3-hydroxy-2,2-dimethylpropyl)-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepine-3-acetic acid

-
-
383662-10-0
ethyl N-[[(3R,5S)-7-chloro-5-(2,3-dimethoxyphenyl)-1-(3-hydroxy-2,2-dimethylpropyl)-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]acetyl]-L-alaninate

| Conditions | Yield |
|---|---|
| With diethylphosphoryl cyanide; triethylamine In N,N-dimethyl-formamide at 20℃; for 0.5h; | 100% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
100-52-7
benzaldehyde

-
-
142128-23-2
(S,E)-ethyl 2-(benzylideneamino)propanoate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 20℃; for 2h; | 100% |
| With triethylamine In ethanol at 20 - 60℃; for 2h; | 100% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
143824-78-6
3-[(S)-2-carboxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)ethyl]indole-1-carboxylic acid tert-butyl ester

-
-
1578249-27-0
C36H39N3O7

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; | 100% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
74-89-5
methylamine

-
-
33194-35-3
(S)-2-amino-N-methyl-propionamide

| Conditions | Yield |
|---|---|
| In water for 0.5h; | 99% |
| In ethanol at 20℃; for 48h; | 90% |
| In ethanol at 20℃; for 48h; | 90% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
772-79-2
4-chlorophenylphosphorodichloridate

-
-
840506-39-0
ethyl N-[chloro(4-chlorophenoxy)phosphoryl]-L-alaninate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -78 - 20℃; | 99% |
| With triethylamine In dichloromethane at -78 - 20℃; | |
| In dichloromethane Alkaline conditions; |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 20h; Reflux; | 99% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
77489-36-2, 81319-58-6, 99945-30-9, 81319-57-5
2,3,6-tri-O-acetyl-4-O-[2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl]-β-D-glucopyranosyl isothiocyanate

-
-
1353744-44-1
N-{[(2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl)-(1→4)-2,3,6-tri-O-acetyl-β-D-glucopyranosylamino]thioxomethyl}alanine ethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In benzene at 20℃; for 5h; | 98.3% |

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 20℃; for 7h; Reflux; | 98% |
| With triethylamine In water at 20℃; for 0.5h; | 86% |
| With sodium hydroxide In dichloromethane at 20℃; for 0.166667h; | 60% |
-
-
619-58-9
4-iodobenzoic acid

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
1072115-61-7
N-(4-iodobenzoyl)-L-alanine ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 4-iodobenzoic acid With benzotriazol-1-ol; 1,2-dichloro-ethane In dichloromethane at 20℃; for 0.166667h; Stage #2: (S)-alanine ethyl ester hydrochloride With triethylamine In dichloromethane at 0 - 20℃; | 98% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
100-39-0
benzyl bromide

-
-
171815-89-7
(S)-ethyl 2-(dibenzylamino)propanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 70℃; for 14h; | 98% |
-
-
1142004-97-4
6-(2-methoxyphenyl)pyrimidin-4-amine

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
1142005-76-2
2-{3-[6-(2-methoxy-phenyl)-pyrimidin-4-yl]-ureido}-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; phenyl chloroformate In 1,4-dioxane; dichloromethane at -78 - 70℃; | 97.8% |

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alanine ethyl ester hydrochloride With triethylamine In dichloromethane Stage #2: t-Boc-L-valine In dichloromethane at 25℃; Inert atmosphere; | 97% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 0℃; |

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-phenyl-2-pentenoic acid With dmap In dichloromethane at 0℃; for 0.25h; Stage #2: (S)-alanine ethyl ester hydrochloride With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 6h; | 97% |
-
-
117-80-6
2,3-Dichloro-1,4-naphthoquinone

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
909797-05-3
(S)-(+)-N-3-(chloro-1,4-naphthoquinon-2-yl)alanine ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 110℃; for 3h; | 96% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
104-94-9
4-methoxy-aniline

-
-
74734-11-5
1H-imidazole-1-carbodithioic acid methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Heating; | 96% |

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
4023-34-1
cyclopropanecarboxylic acid chloride

-
-
885339-39-9
2-(cyclopropanecarbonyl-amino)-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alanine ethyl ester hydrochloride With sodium carbonate Stage #2: N-Cbz-L-Phe With dmap; dicyclohexyl-carbodiimide In dichloromethane at 4 - 20℃; for 1.58333h; | 95% |
| With 2,6-dimethylpyridine; 1-[(1-(cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino)]-uronium hexafluorophosphate In water at 20 - 25℃; | 85% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
598-21-0
2-Bromoacetyl bromide

-
A
-
1127352-23-1
L-2-(2-bromo-acetylamino)-propionic acid ethyl ester

-
B
-
76385-53-0
(S)-N-(2-chloroethanoyl)ethyl alanate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 15h; Inert atmosphere; Overall yield = 67 %; Overall yield = 6.38 g; | A 95% B 5% |

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
14152-97-7
2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl isothiocyanate

-
-
1353744-32-7
N-[(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosylamino)thioxomethyl]alanine ethyl ester

| Conditions | Yield |
|---|---|
| With pyridine In benzene at 20℃; for 5h; | 93.7% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
10382-52-2
2-nitrophenylsulphenyl-L-isoleucine dicyclohexylammonium salt

-
-
97305-67-4
Nps-Ile-Ala-OEt

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In chloroform 1)-10 deg C, 2 h; 2) 2 h, room temperature; | 93% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
619-66-9
4-Carboxybenzaldehyde

-
-
1349769-94-3
C13H15NO4

| Conditions | Yield |
|---|---|
| Stage #1: 4-Carboxybenzaldehyde With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane Stage #2: (S)-alanine ethyl ester hydrochloride In dichloromethane at 0 - 20℃; for 16h; | 93% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
100-52-7
benzaldehyde

-
-
60855-77-8
ethyl N-benzylidenealaninate

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alanine ethyl ester hydrochloride With magnesium sulfate; triethylamine In dichloromethane at 20℃; for 1h; Inert atmosphere; Stage #2: benzaldehyde In dichloromethane at 20℃; Inert atmosphere; | 93% |
| Stage #1: (S)-alanine ethyl ester hydrochloride With triethylamine In dichloromethane at 20℃; Inert atmosphere; Stage #2: benzaldehyde In dichloromethane at 20℃; Inert atmosphere; | |
| In ethanol at 100℃; for 24h; Inert atmosphere; Schlenk technique; Glovebox; | |
| Stage #1: (S)-alanine ethyl ester hydrochloride With sodium sulfate; triethylamine In dichloromethane at 20℃; for 1h; Stage #2: benzaldehyde In dichloromethane |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
71989-23-6
Fmoc-Ile-OH

| Conditions | Yield |
|---|---|
| With O-(N-succinimidyl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In N,N-dimethyl-formamide at 50℃; for 24h; Inert atmosphere; | 93% |
-
-
364-74-9
1,4-difluoro-2-nitrobenzene

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
311346-76-6
ethyl 2-((4-fluoro-2-nitrophenyl)amino)propanoate

| Conditions | Yield |
|---|---|
| With pyridine; triethylamine at 80℃; for 17h; Inert atmosphere; | 92.6% |
| With pyridine; triethylamine at 80℃; for 17h; | 74% |
-
-
3160-59-6
N-Cbz-L-Isoleucine

-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
212070-51-4
Cbz-L-Ile-L-Ala-OEt

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In acetonitrile for 21h; Ambient temperature; | 92% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
770-12-7
O-phenyl phosphorodichloridate

-
-
245078-14-2
(2S)-ethyl 2-(chloro(phenoxy)phosphorylamino)propanoate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 92% |
| With triethylamine In dichloromethane at -78 - 20℃; for 1.5h; | 84% |
| With triethylamine In dichloromethane at -78 - 20℃; for 2.5h; | 75% |
-
-
1115-59-9
(S)-alanine ethyl ester hydrochloride

-
-
110-13-4
2,5-hexanedione

-
-
1325726-63-3
2-(2,5-dimethyl-1H-pyrrol-1-yl)propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alanine ethyl ester hydrochloride With triethylamine In tetrahydrofuran at 20℃; for 0.5h; Paal-Knorr pyrrole synthesis; Stage #2: 2,5-hexanedione With iodine In tetrahydrofuran at 20℃; for 24h; Paal-Knorr pyrrole synthesis; optical yield given as %ee; enantioselective reaction; | 92% |
Ethyl L-alaninate hydrochloride Specification
The Ethyl L-alaninate hydrochloride with CAS registry number of 1115-59-9 is also known as L-Alanine, ethyl ester,hydrochloride (1:1). The IUPAC name is Ethyl (2S)-2-aminopropanoate hydrochloride. It belongs to product categories of Amino Acids; Alanine [Ala, A]; Amino Acids and Derivatives; Amino Hydrochloride; Alanine; Amino Acid Derivatives; Peptide Synthesis. Its EINECS registry number is 214-225-9. In addition, the formula is C5H11NO2.HCl and the molecular weight is 153.61. This chemical is a white to off-white adhering crystalline powder that should be sealed in cool, dry place away from oxidants, water. This chemical may cause inflammation to the skin or other mucous membranes. During using it, avoid contact with skin and eyes.
Physical properties about Ethyl L-alaninate hydrochloride are: (1)ACD/LogP: 0.05; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.38; (4)ACD/LogD (pH 7.4): -0.67; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 4.83; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 4; (12)Flash Point: 3.5 °C; (13)Enthalpy of Vaporization: 36.55 kJ/mol; (14)Boiling Point: 127.8 °C at 760 mmHg; (15)Vapour Pressure: 11 mmHg at 25 °C.
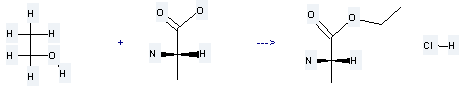
Preparation of Ethyl L-alaninate hydrochloride: it is prepared by reaction of ethanol with L-alanine. The reaction needs reagent SOCl2 and other condition of heating for 2 hours. The yield is about 93%.
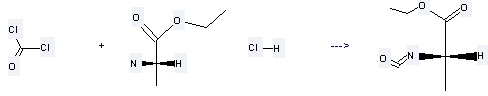
Uses of Ethyl L-alaninate hydrochloride: it is used to produce N-Carbonyl-L-alaninethylester by reaction with carbonyl dichloride. The reaction occurs with reagent pyridine and solvents tetrahydrofuran, CH2Cl2 at the temperature of 0 °C for 2 hours. The yield is about 72%.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CCOC(=O)C(C)N.Cl
2. Isomeric SMILES: CCOC(=O)[C@H](C)N.Cl
3. InChI: InChI=1S/C5H11NO2.ClH/c1-3-8-5(7)4(2)6;/h4H,3,6H2,1-2H3;1H/t4-;/m0./s1
4. InChIKey: JCXLZWMDXJFOOI-WCCKRBBISA-N
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- Ethyl (3R)-piperidine-3-carboxylate
- Ethyl (3R,S)-2,2-difluoro-3-hydroxy-3-(2,2-dimethyldioxolan-4-yl)propionate
- Ethyl (3S)-4-bromo-3-hydroxybutanoate
- Ethyl (3S)-piperidine-3-carboxylate
- Ethyl (3-trifluoromethylbenzoyl)acetate
- 1115-69-1
- 1115-70-4
- 111573-59-2
- 1115-78-2
- 111-57-9
- 111-58-0
- 1115-81-7
- 1115-82-8
- 1115-84-0
- 1115-90-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View