-
Name
Ethyl maltol
- EINECS 225-582-5
- CAS No. 4940-11-8
- Article Data14
- CAS DataBase
- Density 1.261 g/cm3
- Solubility 9.345g/L at 24℃
- Melting Point 85-95 °C(lit.)
- Formula C7H8O3
- Boiling Point 290.3 °C at 760 mmHg
- Molecular Weight 140.139
- Flash Point 124.8 °C
- Transport Information
- Appearance White crystalline powder
- Safety 36
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2-Ethyl-3-hydroxy-4-pyrone;2-Ethyl-3-hydroxy-4H-pyran-4-one;2-Ethylpyromeconic acid;3-Hydroxy-2-ethyl-1,4-pyrone;3-Hydroxy-2-ethyl-4-pyrone;3-Hydroxy-2-ethyl-g-pyrone;
- PSA 50.44000
- LogP 0.90780
Synthetic route
-
-
4208-61-1, 119619-55-5, 127418-31-9, 128948-40-3
ethylfurfuryl alcohol

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

| Conditions | Yield |
|---|---|
| With chlorine In methanol; water 1.) -5 deg C; 2.) 90-95 deg C, 3.5 h; | 67% |
| With chlorine In methanol; water at 90 - 95℃; for 3.5h; Rearrangement; | 67% |
| Stage #1: ethylfurfuryl alcohol With oxygen In methanol at 93℃; Molecular sieve; Stage #2: With oxygen In methanol at 20℃; for 2h; Stage #3: With hydrogenchloride In water at 100℃; for 2h; pH=2.5 - 3; Solvent; Temperature; | 66.8% |
| With tert-butylhypochlorite In acetic acid | 65% |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc In ethanol at 55℃; for 3h; Temperature; |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

| Conditions | Yield |
|---|---|
| With Lawessons reagent In 1,4-dioxane for 1h; Heating; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogen / water / 8 h / 90 °C / 15001.5 Torr 2: chlorine / water; methanol / -5 - 60 °C 3: sodium hydroxide / 3 h / 55 °C 4: zinc; hydrogenchloride / ethanol / 3 h / 55 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: chlorine / water; methanol / -5 - 60 °C 2: sodium hydroxide / 3 h / 55 °C 3: zinc; hydrogenchloride / ethanol / 3 h / 55 °C View Scheme |
-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
100-39-0
benzyl bromide

-
-
111782-87-7
3-(benzyloxy)-2-ethyl-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| In acetone for 6h; Reflux; | 98% |
| Stage #1: 2-ethyl-3-hydroxy-4-pyranone With sodium hydroxide In methanol Heating; Stage #2: benzyl bromide In methanol for 6h; Heating; Further stages.; | 85% |
| With sodium hydroxide In ethanol Heating; | 80% |

| Conditions | Yield |
|---|---|
| In methanol for 1h; Reflux; | 98% |
-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
69-72-7
salicylic acid

-
-
1325228-63-4
2-ethyl-3-hydroxy-4-pyrone/salicylic acid 1:1 cocrystals

| Conditions | Yield |
|---|---|
| In methanol at 64℃; for 1h; | 98% |
-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
74-88-4
methyl iodide

-
-
50741-69-0
2-ethyl-3-methoxy-4Hpyran-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 4h; Inert atmosphere; Reflux; | 97% |
| With potassium carbonate In acetone at 25℃; for 4h; | 93% |
| With potassium carbonate In acetone for 8h; Reflux; |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
124-41-4
sodium methylate

-
-
173413-66-6
Rh(C5(CH3)5)Cl(C5H2O3C2H5)

| Conditions | Yield |
|---|---|
| In methanol byproducts: NaCl; N2-atmosphere; stoich. amts., refluxing for 3 h; solvent removal, extn. into CH2Cl2, filtration (Celite), evapn., recrystn. (CH2Cl2/light petroleum); | 96% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran ethylmaltol stirred with anhyd. THF at room temp., TiCl4 added dropwise to soln., stirred at room. temp for 30 min; filtered, washed with Et2O, recrystd. from CH2Cl2-petroleum ether (1:1); | 95% |
-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
124-41-4
sodium methylate

-
-
151781-33-8
(C6(CH3)3H3)RuCl(OOC5H2C2H5O)

| Conditions | Yield |
|---|---|
| In methanol; water byproducts: NaCl; Sodium methoxide and ethylmaltol are added to a suspn. of the Ru-compd. in CH3OH/H2O and the mixt. is refluxed for 2 h.; The solvent is removed. The residue is dissolved in CH2Cl2. After filtn. the solvent is evaporated, recrystn. from CH2Cl2/diethylether, elem. anal.; | 80% |


| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-3-hydroxy-4-pyranone With lithium hydroxide monohydrate In water at 20℃; for 0.5h; Stage #2: zinc(II) sulfate heptahydrate In water at 20℃; for 5h; | 77% |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

| Conditions | Yield |
|---|---|
| In water VOSO4*3H2O and ethylmaltol (molar ratio 1:2) suspended in water at 45°C; pptd. over 15-20 min; cooled to room temp.; filtered; ppt. air dried; elem. anal.; | 72% |

| Conditions | Yield |
|---|---|
| Stage #1: salicylaldehyde; anthranilic acid hydrazide In methanol for 0.5h; Stage #2: oxovanadium(IV) sulfate; 2-ethyl-3-hydroxy-4-pyranone In methanol for 0.5h; | 72% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water at 160℃; for 12h; pH=5; Autoclave; | 70% |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
960499-98-3
La(ethylmaltol(1-))3

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Ln(NO3)*6H2O was added to soln. ligand in EtOH and heated until ligand completely dissolved, Et3N was added and stirred for 18-24 h; ppt. was filtered, washed with cold water and cold MeOH and dried in vacuo; elem. anal.; | 68% |

| Conditions | Yield |
|---|---|
| Stage #1: bis(acetylacetonate)oxovanadium; 2-ethyl-3-hydroxy-4-pyranone; 2-chloro-N′-(3-ethoxy-2-hydroxybenzylidene)benzohydrazide In methanol at 20℃; for 0.5h; Stage #2: With air In methanol | 68% |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
14254-05-8, 26306-46-7, 130287-64-8
tin(II) n-butoxide

-
-
361148-46-1
Sn(ethylmaltol)2

| Conditions | Yield |
|---|---|
| In toluene a soln. of hydroxyketone in toluene was added a stirred soln. of Sn-compound in toluene under N2, the soln. was stirred for 2 h then left at room temp.; elem. anal.; | 66% |
-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
95790-62-8
2-ethyl-3-hydroxy-5-bromo-4H-pyran-4-one

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane at 95℃; for 18h; Inert atmosphere; | 66% |
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane at 95℃; Inert atmosphere; Schlenk technique; | 32% |
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane at 95℃; for 18h; Inert atmosphere; Schlenk technique; |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

-
-
1829-34-1
2-hydroxy-3-bromobenzaldehyde

-
-
5818-06-4
3-hydroxybenzoic hydrazide

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-3-bromobenzaldehyde; 3-hydroxybenzoic hydrazide In methanol for 0.5h; Stage #2: oxovanadium(IV) sulfate; 2-ethyl-3-hydroxy-4-pyranone In methanol for 0.5h; | 65% |

-
-
4940-11-8
2-ethyl-3-hydroxy-4-pyranone

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Ln(NO3)*6H2O was added to soln. ligand in EtOH and heated until ligand completely dissolved, Et3N was added and stirred for 18-24 h; ppt. was filtered, washed with cold water and cold MeOH and dried in vacuo; elem. anal.; | 64% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-3-hydroxy-4-pyranone; benzyl chloride With sodium hydroxide In methanol; ethanol; water Stage #2: ethanolamine With sodium hydroxide In methanol; ethanol; water | 63% |
| Stage #1: 2-ethyl-3-hydroxy-4-pyranone; benzyl chloride With sodium hydroxide In methanol; water Stage #2: ethanolamine With sodium hydroxide In ethanol; water | 63% |


| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.5h; | 62% |

Ethyl Maltol Chemical Properties
The Molecular Structure of Ethyl maltol (CAS NO.4940-11-8) :
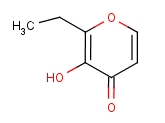
Empirical Formula: C7H8O3
Molecular Weight: 140.1366
IUPAC Name: IUPAC: 2-ethyl-3-hydroxypyran-4-one
Nominal Mass: 140 Da
Average Mass: 140.1366 Da
Monoisotopic Mass: 140.047344 Da
Index of Refraction: 1.54
Molar Refractivity: 34.91 cm3
Molar Volume: 111.1 cm3
Surface Tension: 47 dyne/cm
Density: 1.261 g/cm3
Flash Point: 124.8 °C
Enthalpy of Vaporization: 61.46 kJ/mol
Boiling Point: 290.3 °C at 760 mmHg
Melting Point: 85-95 °C
Vapour Pressure: 0.000228 mmHg at 25°C
InChI
InChI=1/C7H8O3/c1-2-6-7(9)5(8)3-4-10-6/h3-4,9H,2H2,1H3
Smiles
c1(c(ccoc1CC)=O)O
Synonyms: 5-18-01-00135 (Beilstein Handbook Reference) ; BRN 1618110 ; EINECS 225-582-5 ; FEMA No. 3487 ; UNII-L6Q8K29L05
It is a stable white crystalline powder at room temperature and easily dissolves in many polar liquids. Ethyl Maltol (CAS NO.4940-11-8) has a sweet odor that can be described as caramalized sugar and cooked fruit. Ethyl Maltol (CAS NO.4940-11-8) can be easily detected by the human, with as little as 10 parts per million perceivable in air.
Ethyl Maltol Uses
Ethyl Maltol (CAS NO.4940-11-8) is an important flavourant for the food, beverage, and fragrance industry. It is a sweet, synergistic agent used in TOBACCO, food, wines,beverages, essences, cosmetics and other consumables, It has a remarkable effect on improving and increasing the flavor of foods and it enhances the sweetness of foods.
Ethyl Maltol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| chicken | LD50 | oral | 1270mg/kg (1270mg/kg) | Toxicology and Applied Pharmacology. Vol. 15, Pg. 604, 1969. | |
| mouse | LD50 | oral | 780mg/kg (780mg/kg) | Toxicology and Applied Pharmacology. Vol. 15, Pg. 604, 1969. | |
| mouse | LD50 | subcutaneous | 910mg/kg (910mg/kg) | Chemical and Pharmaceutical Bulletin. Vol. 22, Pg. 1008, 1974. | |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 805, 1975. | |
| rat | LD50 | oral | 1150mg/kg (1150mg/kg) | Toxicology and Applied Pharmacology. Vol. 15, Pg. 604, 1969. |
Ethyl Maltol Safety Profile
Hazard Codes:  Xn
Xn
Risk Statements: 22
R22: Harmful if swallowed
Safety Statements: 36
S36: Wear suitable protective clothing
Ethyl Maltol Specification
Ethyl maltol (CAS NO.4940-11-8) is also called as 2-Ethyl pyromeconic acid ; 2-Ethyl-3-hydroxy-4H-pyran-4-one ; 2-Ethylpyromeconic acid ; 3-Hydroxy-2-ethyl-1,4-pyrone ; 3-Hydroxy-2-ethyl-4-pyrone ; 3-Hydroxy-2-ethyl-4H-pyran-4-one ; 3-Hydroxy-2-ethyl-gamma-pyrone ; 4H-Pyran-4-one, 2-ethyl-3-hydroxy- ; Veltol plus ; 2-Ethyl-3-hydroxy-4-pyrone ; 4H-Pyran-4-one, 2-ethyl-3-hydroxy- .
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 4940-39-0
- 494-04-2
- 494-19-9
- 494210-67-2
- 4942-47-6
- 4943-67-3
- 494-38-2
- 4943-86-6
- 4944-60-9
- 494-52-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View