-
Name
Ethyl propiolate
- EINECS 210-795-8
- CAS No. 623-47-2
- Article Data32
- CAS DataBase
- Density 0.998 g/cm3
- Solubility miscible with water
- Melting Point 9 °C
- Formula C5H6O2
- Boiling Point 119.999 °C at 760 mmHg
- Molecular Weight 98.1014
- Flash Point 23.333 °C
- Transport Information UN 3272 3/PG 3
- Appearance clear colorless to pale yellow liquid
- Safety 26-36-37/39-16-33-29-24-23
- Risk Codes 10-36/37/38-36-11
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, F
F
- Synonyms Propiolicacid, ethyl ester (6CI,7CI,8CI);(Ethoxycarbonyl)acetylene;Carboethoxyacetylene;Ethyl 2-propynoate;Ethyl acetylenecarboxylate;Ethylacetylenemonocarboxylate;Ethyl propargylate;Ethylpropynoate;NSC 60551;Propynoic acid ethyl ester;
- PSA 26.30000
- LogP 0.18270
Synthetic route

| Conditions | Yield |
|---|---|
| With sulfuric acid; trimethyl orthoformate In dichloromethane at 40℃; for 24h; Reagent/catalyst; | 86.7% |
| boron trifluoride diethyl etherate 1.) reflux, 1.5 h; 2.) rt., 4 h.; | 60% |
| With sulfuric acid for 24h; Reflux; | 36% |
-
-
38391-86-5
Ethyl half ester of acetylenedicarboxylic acid

-
-
108-88-3
toluene

-
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 100℃; Zeitlicher Verlauf der thermischen Zersetzung; |
-
-
119930-16-4
(E)-4-(Triphenyl-λ5-phosphanylidene)-pent-2-enedioic acid diethyl ester

-
A
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
B
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| equil. react.; |
-
-
38391-86-5
Ethyl half ester of acetylenedicarboxylic acid

-
-
7732-18-5
water

-
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 100℃; Zeitlicher Verlauf der thermischen Zersetzung; |
-
-
527-60-6
Mesitol

-
-
541-41-3
chloroformic acid ethyl ester

-
-
471-25-0
Propiolic acid

-
A
-
1737-55-9
Trifluoressigsaeure-(2,4,6-trimethyl-phenylester)

-
B
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: Propiolic acid With lithium hydride In tetrahydrofuran at 20℃; for 18h; Metallation; Stage #2: chloroformic acid ethyl ester In tetrahydrofuran at -10 - 20℃; Acylation; Stage #3: Mesitol In tetrahydrofuran at 20 - 45℃; Substitution; |

-
-
541-41-3
chloroformic acid ethyl ester

-
-
51114-00-2
sodium 2,4,6-trimethylphenoxide

-
-
471-25-0
Propiolic acid

-
A
-
1737-55-9
Trifluoressigsaeure-(2,4,6-trimethyl-phenylester)

-
D
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: Propiolic acid With lithium hydride In tetrahydrofuran at 20℃; for 18h; Metallation; Stage #2: chloroformic acid ethyl ester In tetrahydrofuran at -10 - 20℃; Acylation; Stage #3: sodium 2,4,6-trimethylphenoxide In tetrahydrofuran at 20 - 45℃; Substitution; |

-
-
764-01-2
methyl propargyl alcohol

-
-
1972-28-7
diethylazodicarboxylate

-
-
108-95-2
phenol

-
A
-
13610-09-8
but-2-ynyloxybenzene

-
B
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triphenylphosphine In tetrahydrofuran; benzene | A 2.18 g (70%) B n/a |

-
-
107-19-7
propargyl alcohol

-
-
1972-28-7
diethylazodicarboxylate

-
-
287108-60-5
5-bromo-2-[(4-hydroxy-benzenesulfonyl)-methyl-amino]-3-methyl-benzoic acid methyl ester

-
A
-
623-47-2
propynoic acid ethyl ester

-
B
-
287108-61-6
5-bromo-3-methyl-2-[methyl-(4-prop-2-ynyloxy-benzenesulfonyl)-amino]-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With triphenylphosphine In tetrahydrofuran; benzene | A n/a B 0.389 g (71%) |

-
-
461695-81-8
(hydrotris(3,5-diisopropylpyrazolyl)borato)CoCCC(O)OCH2CH3

-
-
7732-18-5
water

-
B
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In not given |


| Conditions | Yield |
|---|---|
| Stage #1: acetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.25h; Inert atmosphere; Stage #2: chloroformic acid ethyl ester In tetrahydrofuran; hexane at -78℃; for 8h; Inert atmosphere; | |
| Stage #1: acetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Inert atmosphere; Stage #2: chloroformic acid ethyl ester In tetrahydrofuran; hexane at -78 - 20℃; for 1.5h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With C7H13N2O4P; potassium carbonate In methanol at 20℃; Inert atmosphere; |
-
-
110-89-4
piperidine

-
-
623-47-2
propynoic acid ethyl ester

-
-
81239-00-1
ethyl (E)-3-(piperidin-1-yl)acrylate

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0333333h; Michael addition; | 100% |
| With water at 20℃; for 0.0833333h; optical yield given as %de; stereoselective reaction; | 96% |
| With benzene |

| Conditions | Yield |
|---|---|
| With ammonia at -78 - 20℃; for 5h; | 100% |
| With ammonia at -55℃; for 9h; | 83% |
| With ammonium hydroxide at -10℃; for 1h; Inert atmosphere; | 74% |
-
-
123-75-1
pyrrolidine

-
-
623-47-2
propynoic acid ethyl ester

-
-
65651-80-1
ethyl (E)-3-(pyrrolidin-1-yl)acrylate

| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 16h; | 100% |
| With water In neat (no solvent) at 20℃; for 0.166667h; Michael Addition; Sealed tube; Green chemistry; stereoselective reaction; | 99% |
| With water at 20℃; for 0.0833333h; optical yield given as %de; stereoselective reaction; | 91% |
-
-
110-91-8
morpholine

-
-
623-47-2
propynoic acid ethyl ester

-
-
81239-01-2
ethyl (E)-3-(morpholin-4-yl)acrylate

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0333333h; Michael addition; | 100% |
| With water In neat (no solvent) at 20℃; for 0.166667h; Michael Addition; Sealed tube; Green chemistry; stereoselective reaction; | 99% |
| With water at 20℃; for 0.0833333h; optical yield given as %de; stereoselective reaction; | 94% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
623-47-2
propynoic acid ethyl ester

-
-
115607-78-8
ethyl 3-<(2-methoxyphenyl)amino>acrylate

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With potassium for 12h; | 100% |
| With copper(II) sulfate for 24h; Heating / reflux; | 37% |
-
-
24886-73-5
1-Azidoadamantane

-
-
623-47-2
propynoic acid ethyl ester

-
-
76599-43-4
1-(1-adamantyl)-4-(ethyoxycarbonyl)-1H-1,2,3-triazole

| Conditions | Yield |
|---|---|
| With tetrakis(acetonitrile)copper(I)tetrafluoroborate; tris[(1-benzyl-1H-1,2,3-triazol-4yl)methyl]amine In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 100% |
| With 2,6-dimethylpyridine In water at 20℃; for 8h; Huisgen 1,3-dipolar cycloaddition; regioselective reaction; | 96% |
| With C22H28CuIN2 In neat (no solvent) at 20℃; for 72h; | 81% |
| In toluene at 110℃; for 50h; | 77% |
-
-
7340-17-2
ethyl N-hydroxybenzimidate

-
-
623-47-2
propynoic acid ethyl ester

-
-
139172-68-2, 144946-63-4
ethyl N-<(2'-ethoxycarbonylvinyl)oxy>benzimidate

| Conditions | Yield |
|---|---|
| With triethylamine for 0.166667h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 24h; Ambient temperature; | 100% |
-
-
623-47-2
propynoic acid ethyl ester

-
-
31930-36-6
ethyl (Z)-3-iodopropenoate

| Conditions | Yield |
|---|---|
| With acetic acid; sodium iodide at 70℃; for 16h; Inert atmosphere; | 100% |
| With acetic acid; sodium iodide at 70℃; for 16h; Inert atmosphere; | 99% |
| With acetic acid; sodium iodide | 98% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
623-47-2
propynoic acid ethyl ester

-
-
1112-29-4
ethyl β,β-bis(diethoxyphosphoryl)propionate

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium hydroxide In dichloromethane at 20℃; for 0.0833333h; | 100% |
| With sodium hexamethyldisilazane 1) THF, -50 deg C, 15 min; 2) THF, -50 deg C, 10 min; Yield given. Multistep reaction; | |
| With aluminum oxide; potassium hydroxide at 20℃; for 0.0833333h; Yield given; |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
623-47-2
propynoic acid ethyl ester

-
-
5932-27-4
ethyl 1H-pyrazole-3-carboxylate

| Conditions | Yield |
|---|---|
| In diethyl ether Ambient temperature; | 100% |
-
-
80910-01-6, 108836-41-5, 134733-19-0, 144069-33-0
(S)-2-[(4-methoxybenzyloxy)methyl]oxirane

-
-
623-47-2
propynoic acid ethyl ester

-
-
302554-85-4
ethyl (5S)-5-hydroxy-6-(4-methoxybenzyloxy)hex-2-ynoate

| Conditions | Yield |
|---|---|
| With n-butyllithium; boron trifluoride diethyl etherate In tetrahydrofuran at -78℃; for 0.5h; Ring cleavage; | 100% |
| Stage #1: propynoic acid ethyl ester With n-butyllithium In tetrahydrofuran; hexane at -90℃; for 0.333333h; Stage #2: (S)-2-[(4-methoxybenzyloxy)methyl]oxirane With boron trifluoride diethyl etherate In tetrahydrofuran; hexane at -90 - 0℃; | 100% |
-
-
623-47-2
propynoic acid ethyl ester

-
-
370884-58-5
1-[(2R,3S,6R,7S)-3-Hydroxy-6-(4-methoxy-benzyloxy)-7-(4-methoxy-benzyloxymethyl)-2-methyl-oxepan-2-yl]-propan-2-one

-
-
370884-60-9
(E)-3-[(2R,3S,6R,7S)-6-(4-Methoxy-benzyloxy)-7-(4-methoxy-benzyloxymethyl)-2-methyl-2-(2-oxo-propyl)-oxepan-3-yloxy]-acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In dichloromethane at 20℃; | 100% |
| With 4-methyl-morpholine In dichloromethane at 30℃; for 16h; | 100% |
-
-
623-47-2
propynoic acid ethyl ester

-
-
396714-80-0
1-(benzyloxy)-3-(phenyltellanyl)propan-2-ol

-
-
396714-84-4
(E)-3-(1-benzyloxymethyl-2-phenyltellurenyl-ethoxy)-acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In dichloromethane at 20℃; hetero-Michael addition reaction; | 100% |
| With 4-methyl-morpholine In dichloromethane at 20℃; hetero-Michael addition; | 80% |
-
-
80735-94-0
1-Phenyl-3-buten-1-ol

-
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: propynoic acid ethyl ester With triethylamine In diethyl ether for 0.25h; Inert atmosphere; Stage #2: 1-Phenyl-3-buten-1-ol In diethyl ether for 28h; Inert atmosphere; | 100% |
| Stage #1: propynoic acid ethyl ester With triethylamine In diethyl ether at 20℃; for 0.166667h; Stage #2: 1-Phenyl-3-buten-1-ol In diethyl ether at 20℃; for 48h; | 97% |
-
-
606123-85-7
1-benzylidene-4,4-dimethyl-3-oxopyrazolidin-1-ium-2-ide

-
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With copper(l) iodide; phosphaferrocene-4-(S)-i-Pr-oxazoline; N-Methyldicyclohexylamine In dichloromethane at 20℃; for 20h; | 100% |
-
-
111680-71-8
2-(dimethylamino)-4-oxo-4H-pyrido<1,2-a>pyrimidine-3-carbaldehyde

-
-
623-47-2
propynoic acid ethyl ester

-
-
24461-61-8, 26682-99-5, 37760-98-8, 6591-61-3
phenylglycine methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-(dimethylamino)-4-oxo-4H-pyrido<1,2-a>pyrimidine-3-carbaldehyde; phenylglycine methyl ester In toluene at 85℃; for 1h; Stage #2: propynoic acid ethyl ester In toluene at 85℃; for 3.5h; Stage #3: With pyridinium p-toluenesulfonate In toluene Further stages.; | 100% |

-
-
90-04-0
2-methoxy-phenylamine

-
-
623-47-2
propynoic acid ethyl ester

-
-
142781-90-6
ethyl 3-(2-methoxyphenylamino)acrylate

| Conditions | Yield |
|---|---|
| In ethanol | 100% |
-
-
73183-34-3
bis(pinacol)diborane

-
-
623-47-2
propynoic acid ethyl ester

-
-
1009307-13-4
ethyl (2E)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)acrylate

| Conditions | Yield |
|---|---|
| Stage #1: bis(pinacol)diborane With 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; copper(l) chloride; sodium t-butanolate In tetrahydrofuran at 20℃; for 0.166667h; Schlenk technique; Inert atmosphere; Stage #2: propynoic acid ethyl ester In tetrahydrofuran; methanol Sealed tube; Schlenk technique; Inert atmosphere; | 100% |
| Stage #1: bis(pinacol)diborane With 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; copper(l) chloride; sodium t-butanolate In tetrahydrofuran at 25℃; for 0.166667h; Schlenk technique; Inert atmosphere; Stage #2: propynoic acid ethyl ester In tetrahydrofuran; methanol at 25℃; Schlenk technique; Inert atmosphere; Sealed tube; | 95% |
| Stage #1: bis(pinacol)diborane With 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; copper(l) chloride; sodium t-butanolate In tetrahydrofuran at 25℃; for 0.166667h; Inert atmosphere; Stage #2: propynoic acid ethyl ester In tetrahydrofuran; methanol at 25℃; for 11.4h; Inert atmosphere; | 85.3% |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine at 20℃; for 12h; Michael addition; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: propynoic acid ethyl ester With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: trans-Crotonaldehyde In tetrahydrofuran; hexane at -78℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| With supported Cu(I) bis-2-pyridiylamine functionalized silica Si-BPA*Cu+ In water; tert-butyl alcohol for 1h; Microwave irradiation; regiospecific reaction; | 100% |
| With copper(II) loaded mesoporous SBA-15 In dichloromethane at 20℃; Huisgen Cycloaddition; regioselective reaction; | 64% |
-
-
123-75-1
pyrrolidine

-
-
623-47-2
propynoic acid ethyl ester

-
-
53927-12-1
ethyl-3-(pyrrolidin-1-yl)prop-2-enoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 10 - 25℃; for 1h; | 100% |
| In acetonitrile at 20℃; for 10h; | 98% |
| In acetonitrile at 20℃; Inert atmosphere; | |
| In acetonitrile at 20℃; | 9.47 g |
-
-
105310-97-2
N-benzyl-α-diazoacetamide

-
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 22℃; under 760.051 Torr; | 100% |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In dichloromethane at 0 - 20℃; for 4h; | 100% |
-
-
95239-02-4
C10H16N2O4

-
-
7518-21-0
2-(1-methyl-1H-indol-3-yl)ethylamine

-
-
623-47-2
propynoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-(1-methyl-1H-indol-3-yl)ethylamine; propynoic acid ethyl ester In dichloromethane at 20℃; Stage #2: C10H16N2O4 In dichloromethane; toluene for 2h; Reflux; Stage #3: With trifluoroacetic acid In dichloromethane; toluene Reflux; | 100% |


| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In butan-1-ol; benzene at 40℃; for 8h; regioselective reaction; | 99.1% |
| With ClO4 In methanol Ambient temperature; | 87% |
| With piperazine; LACTIC ACID In N,N-dimethyl-formamide at 90℃; for 12h; | 78% |
-
-
623-47-2
propynoic acid ethyl ester

-
-
26631-66-3
(Z)-ethyl 2,3-dibromoprop-2-enoate

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 70℃; for 0.5h; | 99% |
| With bromine In tetrachloromethane at 70℃; for 2.5h; | 90% |
| With bromine In tetrachloromethane at 70℃; for 1.5h; | 83% |
| With bromine In tetrachloromethane Irradiation; | |
| With bromine In tetrachloromethane at 70℃; for 1.5h; Yield given; |
-
-
622-37-7
Phenyl azide

-
-
623-47-2
propynoic acid ethyl ester

-
-
4915-97-3
1-phenyl-1H-[1,2,3]triazole-4-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With C30H23Cl2N7O4Ru(1+)*Cl(1-) In water at 23℃; for 0.0666667h; Huisgen Cycloaddition; Sonication; Green chemistry; regioselective reaction; | 99% |
| In ethanol for 20h; Heating; | 95% |
| With copper(I) oxide In water at 25℃; for 0.0833333h; Inert atmosphere; | 95% |
Ethyl propiolate Chemical Properties
The Molecular Weight of Ethyl propiolate(623-47-2): 98.1
Molecular Structure :
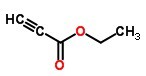
EINECS: 210-795-8
Boiling Point: 120 °C at 760 mmHg
Flash Point: 23.3 °C
Water Solubility miscible
Index of Refraction: 1.418
Molar Refractivity: 24.81 cm3
Molar Volume: 98.3 cm3
Polarizability: 9.83 10-24 cm3
Surface Tension: 32.5 dyne/cm
Density: 0.997 g/cm3
Enthalpy of Vaporization: 35.81 kJ/mol
Vapour Pressure: 15.5 mmHg at 25°C
storage temp.: 2-8°C
IUPAC Name: ethyl prop-2-ynoate
Synonyms: ACETYLENECARBOXYLIC ACID ETHYL ESTER;ETHYL PROPIOLATE;2-Propynoic acid, ethyl ester;Ethyl 2-propynoate;Ethyl propynoate;ETHYL ACETYLENECARBOXYLATE;EPL;(Ethoxycarbonyl)acetylene;
Ethyl propiolate Uses
Ethyl propiolate Safety Profile
 Xi,
Xi,  F
F Hazard Note: Irritant
HazardClass: 3
The Risk Statements information of Ethyl propiolate(623-47-2):
10: Flammable
11: Highly Flammable
36: Irritating to the eyes
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of Ethyl propiolate(623-47-2):
16: Keep away from sources of ignition - No smoking
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24: Avoid contact with skin
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37/39: Wear suitable gloves and eye/face protection
RIDADR: UN 3272 3/PG 3
WGK Germany: 3
PackingGroup: II
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 62348-13-4
- 623-48-3
- 623-49-4
- 623-50-7
- 62351-47-7
- 623-51-8
- 623-53-0
- 62353-75-7
- 62356-27-8
- 623-56-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View