-
Name
Etodolac
- EINECS 629-689-1
- CAS No. 41340-25-4
- Article Data11
- CAS DataBase
- Density 1.193 g/cm3
- Solubility 40mg/L(37 oC)
- Melting Point 145-148 °C
- Formula C17H21NO3
- Boiling Point 507.9 °C at 760 mmHg
- Molecular Weight 287.359
- Flash Point 260.9 °C
- Transport Information UN 3249
- Appearance crystalline solid
- Safety 22-36-45-26-36/37
- Risk Codes 23/24/25-40-36-25-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T,
T,  Xi
Xi
- Synonyms (1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetic acid;1,3,4,9-Tetrahydro-1,8-diethylpyrano(3,4-b)indole-1-acetic acid;Prestwick_209;Pyrano(3,4-b)indole-1-acetic acid, 1,8-diethyl-1,3,4,9-tetrahydro-;Lodine;Lodine (TN);Ultradol;{Pyrano[3,} 4-b]indole-1-acetic acid, 1,8-diethyl-1,3,4,9-tetrahydro-;{1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic} acid;Etodolacum [INN-Latin];1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic acid;Etodolaco [INN-Spanish];Pyrano(3,4-b)indole-1-acetic acid, 1,3,4,9-tetrahydro-1,8-diethyl-;Pyrano[3,4-b]indole-1-acetic acid,1,8-diethyl-1,3,4,9-tetrahydro-;Etodolac (JAN/USP);Etodolic acid;AY 24236;Etodolac Acid;
- PSA 62.32000
- LogP 3.38300
Synthetic route

| Conditions | Yield |
|---|---|
| (i) TsOH, benzene, (ii) aq. NaOH, EtOH; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With Lipase QL In di-isopropyl ether; water for 192h; Product distribution; Ambient temperature; various lipase enzymes; |
-
-
87226-41-3
(R)-etodolac

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With tin(IV) chloride In tetrahydrofuran; dichloromethane for 4h; Heating / reflux; | |
| sulfuric acid In isopropyl alcohol at 20℃; for 23h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With tin(IV) chloride In dichloromethane at 20℃; for 2h; | |
| Stage #1: (R)-etodolac; (S)-etodolac In dichloromethane at 20℃; for 2h; Stage #2: With sodium hydroxide; formic acid at 0℃; for 1h; pH=5; |
-
-
87249-11-4
(S)-etodolac

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| sulfuric acid In isopropyl alcohol at 20℃; for 4 - 18h; Product distribution / selectivity; | |
| boron trifluoride diethyl etherate In isopropyl alcohol at 20℃; for 15h; Product distribution / selectivity; |
-
-
41340-36-7
7-ethyl-2-(1H-indol-3-yl)ethan-1-ol

-
-
30414-53-0
methyl propanoyl acetate

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol Pictet-Spengler Synthesis; |
-
-
41340-25-4
etodolac

-
-
200880-25-7
(+/-)-2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)ethanol

| Conditions | Yield |
|---|---|
| With LiAlH4 In tetrahydrofuran; hexane; ethyl acetate | 98% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 12h; | 92% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 20℃; Cooling with ice; | 96% |
-
-
528-43-8
Magnolol

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1h; | 90% |
| With dmap In dichloromethane at 25 - 30℃; | 90% |
-
-
64-19-7
acetic acid

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethylethylenediamine; etodolac With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In acetonitrile at 20℃; for 3h; Stage #2: acetic acid In ethyl acetate | 89.4% |

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With dmap; copper diacetate In acetonitrile at 80℃; for 3h; Schlenk technique; Sealed tube; | 86% |
-
-
67-56-1
methanol

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With sulfuric acid for 8h; Reflux; | 85% |
| With sulfuric acid for 3h; Reflux; | 66% |
| With sulfuric acid | |
| With sulfuric acid for 3h; Reflux; |
-
-
115423-27-3
3-hydroxypropyl selenocyanate

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| Stage #1: etodolac In N,N-dimethyl-formamide at 25℃; for 0.5h; Stage #2: 3-hydroxypropyl selenocyanate In N,N-dimethyl-formamide at 25℃; for 16h; | 85% |
-
-
89-83-8
thymol

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 25℃; for 72h; Steglich Esterification; | 83.5% |
-
-
41340-25-4
etodolac

-
-
591752-31-7
2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: etodolac With oxalyl dichloride at 20℃; Stage #2: With ammonium hydroxide | 82.5% |
-
-
458-37-7
curcumin

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1h; | 80% |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 25 - 30℃; | A 56% B 80% |

-
-
528-43-8
Magnolol

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1h; | 78% |
| With dmap In dichloromethane at 25 - 30℃; | 78% |
-
-
100-39-0
benzyl bromide

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 50℃; for 2h; | 73% |

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| Stage #1: etodolac With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane; N,N-dimethyl-formamide at 25℃; for 0.5h; Stage #2: 1-selenocyano-2-ethylamine hydrobromide In dichloromethane; N,N-dimethyl-formamide at 25℃; for 16h; | 72% |
-
-
1816-88-2
2-azido-1-phenylethan-1-one

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 110℃; for 10h; Sealed tube; | 72% |

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| Stage #1: etodolac With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane; N,N-dimethyl-formamide at 25℃; for 0.5h; Inert atmosphere; Stage #2: 2-selenocyanatopropanamine hydrobromide In dichloromethane; N,N-dimethyl-formamide at 25℃; for 16h; Inert atmosphere; | 65% |
| Stage #1: etodolac With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane; N,N-dimethyl-formamide at 25℃; for 0.5h; Stage #2: 2-selenocyanatopropanamine hydrobromide In dichloromethane; N,N-dimethyl-formamide at 25℃; for 16h; | 60% |
-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; Inert atmosphere; | 59% |
-
-
38780-40-4, 52691-24-4, 61848-70-2, 61848-66-6, 61848-62-2
{(1R,2R)-cyclohexane-1,2-diamine}dichloridoplatinum(II)

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With sodium carbonate; silver nitrate In N,N-dimethyl-formamide at 20℃; for 24h; Darkness; | 52% |
-
-
19408-84-5
dihydrocapsaicin

-
-
41340-25-4
etodolac

-
-
1224429-03-1
(1,8-diethyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indol-1-yl)-acetic acid 2-methoxy-4-[(8-methyl-nonanoylamino)-methyl]-phenyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 30 - 35℃; | 49.6% |

| Conditions | Yield |
|---|---|
| Stage #1: etodolac With triethylamine In tetrahydrofuran at -50℃; for 1h; Inert atmosphere; Stage #2: Propargylamine With isobutyl chloroformate In tetrahydrofuran at -50 - 20℃; for 13h; Inert atmosphere; | 46.8% |
-
-
1336-21-6
ammonium hydroxide

-
-
79-37-8
oxalyl dichloride

-
-
41340-25-4
etodolac

-
-
591752-31-7
2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetamide

| Conditions | Yield |
|---|---|
| N,N-dimethyl-formamide In tetrahydrofuran; hexane; dichloromethane; ethyl acetate | 35% |


| Conditions | Yield |
|---|---|
| Stage #1: etodolac With 1,1'-carbonyldiimidazole Inert atmosphere; Stage #2: potassium ethyl methylmalonate With magnesium chloride Inert atmosphere; | 35% |
-
-
404-86-4
capsaicin

-
-
41340-25-4
etodolac

-
-
1224429-01-9
(1,8-diethyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indol-1-yl)-acetic acid 2-methoxy-4-[(8-methyl-non-6-enoylamino)-methyl]-phenyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 30 - 35℃; | 30.2% |
-
-
41340-25-4
etodolac

-
A
-
111478-86-5
1,8-diethyl-4-oxo-1,3,4,9-tetrahydropyrano[3,4-b]-indole-1-acetic acid

| Conditions | Yield |
|---|---|
| With iodosylbenzene; Cl8TTPFe(III)Cl In dichloromethane at 20℃; for 2h; | A 20% B 25% |
-
-
41340-25-4
etodolac

-
-
74-88-4
methyl iodide

-
A
-
114737-77-8
3a,10-diethyl-5,6,6a,11-tetrahydro-6a-methylfuro<3',2'2,3>pyrano<3,4-b>indol-2(3H)-one

-
B
-
114720-05-7
2-(1,8-diethyl-9-methyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indol-1-yl)acetic acid

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 50℃; for 2h; | A 20% B 16% |

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| In methanol for 72h; Heating; pH 6.0; | 20% |
-
-
125074-46-6, 31246-62-5, 31246-63-6, 31246-66-9, 53261-25-9
(OC-6-33)-(diammine)dichloridodihydroxidoplatinum(IV)

-
-
41340-25-4
etodolac

| Conditions | Yield |
|---|---|
| With triethylamine; O‐(1H‐benzotriazol‐1‐yl)‐N,N,N′,N′‐tetramethyluronium tetrafluoroborate In N,N-dimethyl-formamide at 20℃; for 24h; Darkness; | 19.5% |
-
-
41340-25-4
etodolac

-
A
-
41340-36-7
7-ethyl-2-(1H-indol-3-yl)ethan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 85℃; for 18h; Product distribution; Rate constant; Thermodynamic data; Ea, ΔS<*>, mechanism of etodolac degradation in aqueous solutionsat various temperatures (75-905 deg C), time and pH, effect of pH and temperature, catalytic effect of buffer system; |

-
-
41340-25-4
etodolac

-
-
87249-11-4
(+)-etodolac
Etodolac Chemical Properties
Molecule structure of Etodolac (CAS NO.41340-25-4):
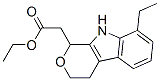
IUPAC Name: 2-(1,8-Diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl)acetic acid
Molecular Weight: 287.35354 g/mol
Molecular Formula: C17H21NO3
Density: 1.193 g/cm3
Melting Point:145-148 °C
Boiling Point: 507.9 °C at 760 mmHg
Flash Point: 260.9 °C
Index of Refraction: 1.596
Molar Refractivity: 81.98 cm3
Molar Volume: 240.7 cm3
Surface Tension: 53.9 dyne/cm
Enthalpy of Vaporization: 81.93 kJ/mol
Vapour Pressure: 3.88E-11 mmHg at 25 °C
Storage Temp.: 2-8 °C
XLogP3-AA: 2.8
H-Bond Donor: 2
H-Bond Acceptor: 3
Rotatable Bond Count: 4
Exact Mass: 287.152144
MonoIsotopic Mass: 287.152144
Topological Polar Surface Area: 62.3
Heavy Atom Count: 21
Canonical SMILES: CCC1=CC=CC2=C1NC3=C2CCOC3(CC)CC(=O)O
InChI: InChI=1S/C17H21NO3/c1-3-11-6-5-7-12-13-8-9-21-17(4-2,0-14(19)20)16(13)18-15(11)12/h5-7,18H,3-4,8-10H2,1-2H3,(H,19,20)
InChIKey: NNYBQONXHNTVIJ-UHFFFAOYSA-N
Product Categories: APIs; Intermediates & Fine Chemicals; Pharmaceuticals
Etodolac Uses
Etodolac (CAS NO.41340-25-4) is a non-steroidal anti-inflammatory drug (NSAID). It is used to anti-inflammatory.
Etodolac Production
Etodolac is manufactured by Shire under the trade name Lodine SR and by Meda Pharmaceuticals under the name Eccoxolac . Non-propriety etodolac is also available.
Etodolac Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | oral | > 4gm/kg (4000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: FOOD INTAKE (ANIMAL) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Oyo Yakuri. Pharmacometrics. Vol. 40, Pg. 469, 1990. |
| mouse | LD50 | intraperitoneal | 338mg/kg (338mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Oyo Yakuri. Pharmacometrics. Vol. 40, Pg. 469, 1990. |
| mouse | LD50 | oral | 593mg/kg (593mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Oyo Yakuri. Pharmacometrics. Vol. 40, Pg. 469, 1990. |
| mouse | LD50 | subcutaneous | 502mg/kg (502mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Oyo Yakuri. Pharmacometrics. Vol. 40, Pg. 469, 1990. |
| rat | LD50 | intraperitoneal | 100mg/kg (100mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Oyo Yakuri. Pharmacometrics. Vol. 40, Pg. 469, 1990. |
| rat | LD50 | oral | 94mg/kg (94mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Oyo Yakuri. Pharmacometrics. Vol. 40, Pg. 469, 1990. |
| rat | LD50 | subcutaneous | 113mg/kg (113mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Oyo Yakuri. Pharmacometrics. Vol. 40, Pg. 469, 1990. |
Etodolac Safety Profile
Hazard Codes:  T,
T,  Xi
Xi
Risk Statements: 23/24/25-40-36-25-36/37/38
R23/24/25: Toxic by inhalation, in contact with skin and if swallowed.
R40: Limited evidence of a carcinogenic effect.
R36: Irritating to eyes.
R25: Toxic if swallowed.
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 22-36-45-26-36/37
S22: Do not breathe dust.
S36: Wear suitable protective clothing.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37: Wear suitable protective clothing and gloves.
RIDADR: 3249
WGK Germany: 3
RTECS: UQ0360000
HazardClass: 6.1(b)
PackingGroup: III
Etodolac Specification
Etodolac (CAS NO.41340-25-4) is also named as (+-)-1,8-Diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic acid ; 1,3,4,9-Tetrahydro-1,8-diethylpyrano(3,4-b)indole-1-acetic acid ; 1,8-Diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic acid ; AY 24236 ; CCRIS 3923 ; Etodolaco ; Etodolaco [INN-Spanish] ; Etodolacum ; Etodolacum [INN-Latin] ; Etodolic acid ; Lodine ; Lodine XL ; NSC 282126 ; Pyrano(3,4-b)indole-1-acetic acid, 1,8-diethyl-1,3,4,9-tetrahydro-(+-)- ; UNII-2M36281008 ; Ultradol . Etodolac (CAS NO.41340-25-4) is crystalline solid. Etodolac belongs to a class of drugs called nonsteroidal anti-inflammatory drugs (NSAIDs).
Related Products
- Etodolac
- Etodolac methyl ester
- Etodolac methyl ester
- 41340-30-1
- 41340-31-2
- 41340-36-7
- 41340-78-7
- 41340-88-9
- 41342-53-4
- 41344-25-6
- 4134-56-9
- 41351-30-8
- 41354-03-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View