-
Name
16-HYDROXYHEXADECANOIC ACID
- EINECS 208-028-7
- CAS No. 506-13-8
- Article Data38
- CAS DataBase
- Density 0.956g/cm3
- Solubility
- Melting Point 94-98 °C(lit.)
- Formula C16H32O3
- Boiling Point 414.4 °C at 760 mmHg
- Molecular Weight 272.428
- Flash Point 218.6 °C
- Transport Information
- Appearance white crystalline powder
- Safety 22-24/25
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms Junipericacid (6CI);16-Hydroxyhexadecanoic acid;16-Hydroxypalmitic acid;Lanopalmiticacid;NSC 159292;Palmitic acid, 16-hydroxy-;w-Hydroxyhexadecanoic acid;w-Hydroxypalmitic acid;
- PSA 57.53000
- LogP 4.52470
Synthetic route
-
-
1619-68-7, 17278-80-7, 18951-79-6
16-hydroxy-9-hexadecenoic acid

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal | 100% |
| With hydrogen; palladium on activated charcoal |
-
-
91544-43-3
16-Hydroxy-hexadeca-10,12-diynoic acid

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In methanol under 760 Torr; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With water; tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In toluene at 95℃; for 6h; | 97% |
| With potassium hydroxide; polystyrene-CH2=O(CH2CH2O)6.4H copolymer In hexane at 70℃; for 5h; | 96% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In benzene at 70℃; | 85% |
-
-
14024-18-1
iron(III)-acetylacetonate

-
-
36653-82-4
1-Hexadecanol

-
A
-
56072-26-5
4-hydroxy-5-methylpentan-2-one

-
B
-
629-70-9
hexadecyl acetate

-
C
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| at 80 - 300℃; under 760.051 Torr; for 2h; Green chemistry; | A n/a B n/a C n/a D 95% |


| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol | 80% |
-
-
109-29-5
15-hexadecanolide

-
-
112-90-3
(Z)-9-octadecen-1-amine

-
A
-
506-13-8
16-hydroxyhexadecanoic acid

-
B
-
1220909-16-9
N-oleyl-16-hydroxyhexadecanoylamine

| Conditions | Yield |
|---|---|
| With lipase PS from Pseudomonas cepacia; water In Hexadecane at 60℃; for 24h; Miniemulsion system; Enzymatic reaction; | A 77% B 14% |


| Conditions | Yield |
|---|---|
| (microbiological transformation); |
-
-
627-91-8
adipic acid monomethyl ester

-
-
100912-48-9
12-acetoxydodecanoic acid

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| (i) Na, MeOH, (electrolysis), (ii) KOH, EtOH; Multistep reaction; |
-
-
1593-55-1
azelaic acid monoethyl ester

-
-
30993-88-5
9-acetoxy nonanoic acid

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| (i) (electrochemical oxidation), aq. K2CO3, (ii) KOH, EtOH; Multistep reaction; |

| Conditions | Yield |
|---|---|
| (i) (electrochemical oxidation), aq. K2CO3, (ii) KOH, EtOH; Multistep reaction; |
-
-
65119-97-3
heptadec-16-enoic acid

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| (i) O3, (ii) NaBH4, KOH; Multistep reaction; |
-
-
36575-67-4
16-hydroxyhexadecanoic acid methyl ester

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| (saponification); |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; mercury; zinc |
-
-
93158-27-1
16-Hydroxy-hexadecin-(12)-saeure

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol |
-
-
112657-63-3
16-Acetoxy-8-oxo-hexadecanoic acid methyl ester

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrazine hydrate In diethylene glycol |
-
-
112553-37-4
16-Acetoxy-10-oxo-hexadecansaeure-(1)-methylester

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrazine hydrate In diethylene glycol |
-
-
18941-99-6
16-Hydroxy-hexadecin-(9)-saeure

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In diethyl ether Yield given; |
-
-
103687-90-7
16-(Tetrahydro-pyran-2-yloxy)-hexadecanenitrile

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; toluene-4-sulfonic acid 1.) methanol; 2.) reflux; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| durch Verseifen; |

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With acetic acid; platinum | |
| With acetic acid; platinum |

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With chromic acid; acetic acid beim Verseifen der erhaltenen Acetyljuniperinsaeure; |

| Conditions | Yield |
|---|---|
| unter Druck.Hydrogenation; |


-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| durch Verseifen; |

-
A
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With ethanol; sodium |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) 1.6 M BuLi / 1.) THF, hexane, 10 deg C, HMPT, 1 h; 2.) THF, 20-25 deg C 2: 96 percent / hydrogen / PtO2 / ethyl acetate / 15 h 3: 89 percent / tributylamine / 24 h / Heating 4: 1.) TsOH; 2.) 10 N NaOH / 1.) methanol; 2.) reflux View Scheme |
-
-
103687-89-4
ω-bromo-1-[(tetrahydro-2H-pyran-2-yl)oxy]pentadecane

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 89 percent / tributylamine / 24 h / Heating 2: 1.) TsOH; 2.) 10 N NaOH / 1.) methanol; 2.) reflux View Scheme |
-
-
103687-88-3
2-(15-Bromo-pentadec-2-ynyloxy)-tetrahydro-pyran

-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 96 percent / hydrogen / PtO2 / ethyl acetate / 15 h 2: 89 percent / tributylamine / 24 h / Heating 3: 1.) TsOH; 2.) 10 N NaOH / 1.) methanol; 2.) reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: NaOEt / ethanol; diethyl ether / 48 h / Ambient temperature 2: 48percent HBr / H2O / 4 h / 120 - 150 °C 3: 76 percent / LiNH2, Fe(NO3)3 / tetrahydrofuran; NH3 / 20 h 4: H2 / PtO2 / diethyl ether View Scheme |
-
-
67-56-1
methanol

-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
36575-67-4
16-hydroxyhexadecanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With acetyl chloride | 100% |
| With toluene-4-sulfonic acid | 100% |
| With toluene-4-sulfonic acid at 20℃; for 16h; Inert atmosphere; | 100% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
18297-63-7
N,N'-bis(trimethylsilyl)urea

| Conditions | Yield |
|---|---|
| tetrabutyl ammonium fluoride In dichloromethane at 0℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Reflux; | 100% |
| In chloroform at 70℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide In acetic acid for 72h; Heating; | 99% |
| With hydrogen bromide In acetic acid for 72h; Substitution; Heating; | 99% |
| With sodium hydroxide; sulfuric acid; nitrogen; hydrogen bromide In acetic acid | 88% |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; sodium iodide In acetonitrile | 98% |
| With chloro-trimethyl-silane; sodium iodide In acetonitrile at 70℃; for 1.5h; | 97% |
| With phosphorus; iodine |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
106-95-6
allyl bromide

-
-
275808-63-4
16-hydroxyhexadecanoic acid allyl ester

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 23℃; for 48h; Addition; | 98% |

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In tetrahydrofuran; chloroform for 18h; Reflux; Inert atmosphere; | 97% |
| With dmap; 4-(dimethylamino)pyridine hydrochloride; dicyclohexyl-carbodiimide In tetrahydrofuran; chloroform Heating; | 96% |
| With dmap; polymer bound carbodiimide; 4-(dimethylamino)pyridine hydrochloride In tetrahydrofuran; chloroform Cyclization; lactonisation; Heating; | 96% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
40615-36-9
4,4'-dimethoxytrityl chloride

| Conditions | Yield |
|---|---|
| With pyridine; triethylamine at 20℃; for 3h; Inert atmosphere; | 97% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
36575-67-4
16-hydroxyhexadecanoic acid methyl ester

| Conditions | Yield |
|---|---|
| In methanol | 95% |
| In diethyl ether at 0℃; for 2h; | 95% |
| In dichloromethane |

| Conditions | Yield |
|---|---|
| With acetyl chloride at 20℃; for 4h; | 94% |
| With acetyl chloride Heating; |
-
-
1221151-98-9
2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl ortho-(hex-1-ynyl)benzoate

-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
1221152-26-6
C50H58O12

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; trifluoromethanesulfonyloxy(triphenylphosphine)gold(I) In dichloromethane at 20℃; Molecular sieve; Inert atmosphere; chemoselective reaction; | 94% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
75-36-5
acetyl chloride

-
-
85258-69-1
ethyl 16-hydroxyhexadecanoate

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 4h; | 94% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
A
-
109-29-5
15-hexadecanolide

-
B
-
660-65-1
1,18-dioxo-17,34-dioxacyclotetratricontane

| Conditions | Yield |
|---|---|
| With p-nitrobenzoic anhydride; scandium tris(trifluoromethanesulfonate) In tetrahydrofuran; acetonitrile Heating; Yields of byproduct given; | A 92% B n/a |
| With dmap; iodine; di-2-thienyl carbonate In toluene; acetonitrile for 10h; Heating; | A 92% B n/a |
| With dmap; di-2-thienyl carbonate; hafnium(IV) trifluoromethanesulfonate In toluene; acetonitrile at 100℃; for 5h; | A 91% B n/a |

| Conditions | Yield |
|---|---|
| With mesoporous silica HMS2-SO3H at 110℃; for 15h; Product distribution; Further Variations:; Reagents; reaction times; | 91% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane; N,N-dimethyl-formamide at 20℃; for 24h; |
-
-
1230076-22-8
2,3,4-tri-O-acetyl-L-rhamnopyranosyl ortho-hexynylbenzoate

-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
1221152-45-9
C28H48O10

| Conditions | Yield |
|---|---|
| With trifluoromethanesulfonyloxy(triphenylphosphine)gold(I); boron trifluoride diethyl etherate; 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; Molecular sieve; Inert atmosphere; chemoselective reaction; | 90% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
75-08-1
ethanethiol

-
-
66921-94-6
16-hydroxy-hexadecanethioic acid S-ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 10h; | 87% |
| With dmap; dicyclohexyl-carbodiimide |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
93472-41-4
C22H48O3Si2

| Conditions | Yield |
|---|---|
| With pyridine; chloro-trimethyl-silane In dichloromethane at 60℃; | 87% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
98-88-4
benzoyl chloride

-
-
173288-97-6
16-benzoyloxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 0.5h; Ambient temperature; | 87% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; immobilized lipase from Candida antarctica (CA; Novozym 435) In toluene at 70℃; under 140 - 150 Torr; for 6h; Enzymatic reaction; | 85% |
-
-
1221151-98-9
2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl ortho-(hex-1-ynyl)benzoate

-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
1221152-23-3
C50H58O12

| Conditions | Yield |
|---|---|
| With trifluoromethanesulfonyloxy(triphenylphosphine)gold(I); boron trifluoride diethyl etherate; 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; Molecular sieve; Inert atmosphere; chemoselective reaction; | 84% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
288851-53-6
16-hydroxy-N-methoxy-N-methylhexadecanamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 83% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 25℃; for 2h; | 78% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
31828-68-9
L-homocysteine thiolactone hydrochloride

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 72h; Molecular sieve; | 78% |
-
-
506-13-8
16-hydroxyhexadecanoic acid

-
-
58479-61-1
tert-butylchlorodiphenylsilane

| Conditions | Yield |
|---|---|
| With pyridine; 1H-imidazole; triethylamine In dichloromethane; N,N-dimethyl-formamide; toluene at 20℃; for 2h; Inert atmosphere; | 78% |
Hexadecanoic acid,16-hydroxy- Specification
The Hexadecanoic acid,16-hydroxy-, with CAS registry number 506-13-8, belongs to the following product categories: (1)omega-Functional Alkanols, Carboxylic Acids, Amines & Halides; (2)omega-Hydroxycarboxylic Acids. It has the systematic name of 16-hydroxyhexadecanoic acid. And the chemical formula of this chemical is C16H32O3. This chemical is a kind of white crystalline powder. It should be stored at the temperature of 2-8°C.
Physical properties of Hexadecanoic acid,16-hydroxy-: (1)ACD/LogP: 5.15; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 4.35; (4)ACD/LogD (pH 7.4): 2.56; (5)ACD/BCF (pH 5.5): 769.52; (6)ACD/BCF (pH 7.4): 12.35; (7)ACD/KOC (pH 5.5): 2405.94; (8)ACD/KOC (pH 7.4): 38.6; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 16; (12)Polar Surface Area: 35.53 Å2; (13)Index of Refraction: 1.468; (14)Molar Refractivity: 79.27 cm3; (15)Molar Volume: 284.7 cm3; (16)Polarizability: 31.42×10-24cm3; (17)Surface Tension: 38 dyne/cm; (18)Density: 0.956 g/cm3; (19)Flash Point: 218.6 °C; (20)Enthalpy of Vaporization: 77.1 kJ/mol; (21)Boiling Point: 414.4 °C at 760 mmHg; (22)Vapour Pressure: 1.34E-08 mmHg at 25°C.
Preparation: this chemical can be prepared by oxacycloheptadecan-2-one. This reaction will need reagent aq. NaOH and solvent ethanol.
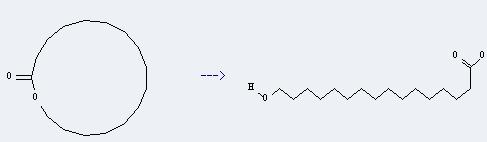
Uses of Hexadecanoic acid,16-hydroxy-: it can be used to produce oxacycloheptadecan-2-one. This reaction will need solvent heptane. The reaction time is 8 hour(s) with reaction temperature of 40 ℃. The yield is about 50%.
.jpg)
When you are using this chemical, please be cautious about it as the following:
The Hexadecanoic acid,16-hydroxy- irritates to eyes, respiratory system and skin. When use it, do not breathe dust and avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)CCCCCCCCCCCCCCCO
(2)InChI: InChI=1/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
(3)InChIKey: UGAGPNKCDRTDHP-UHFFFAOYAB
(4)Std. InChI: InChI=1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
(5)Std. InChIKey: UGAGPNKCDRTDHP-UHFFFAOYSA-N
Related Products
- Hexadecanoic acid, 1,1'-[1-[(phosphonooxy)methyl]-1,2-ethanediyl] ester, sodium salt (1:2)
- Hexadecanoic acid, 16-bromo-, methyl ester
- Hexadecanoic acid, 3-hydroxy-
- Hexadecanoic acid, hentriacontafluoro-
- Hexadecanoic acid, isononyl ester(9CI)
- Hexadecanoic acid, propyl ester
- Hexadecanoic acid, tin(2+) salt (2:1)
- Hexadecanoic acid,1,1',1''-(1,2,3-propanetriyl) ester
- Hexadecanoic acid,1,1'-[(1S)-1-(hydroxymethyl)-1,2-ethanediyl] ester
- Hexadecanoic acid,1,1'-[1-(hydroxymethyl)-1,2-ethanediyl] ester
- 50614-84-1
- 50615-18-4
- 50615-66-2
- 50621-08-4
- 506-21-8
- 50622-09-8
- 50623-73-9
- 506-24-1
- 50624-46-9
- 506-26-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View