-
Name
Isobutyryl chloride
- EINECS 201-194-1
- CAS No. 79-30-1
- Article Data87
- CAS DataBase
- Density 1.03 g/cm3
- Solubility soluble in ether
- Melting Point -90 °C
- Formula C4H7ClO
- Boiling Point 90.9 °C at 760 mmHg
- Molecular Weight 106.552
- Flash Point 1.1 °C
- Transport Information UN 2395 3/PG 2
- Appearance Colorless liquid
- Safety 16-23-26-36-45-36/37/39-28A
- Risk Codes 11-35-23
-
Molecular Structure
-
Hazard Symbols
 F,
F, C,
C, T
T
- Synonyms alpha-Methylpropionyl chloride;2-Methylpropionyl chloride;Isobutyryl chloride [UN2395] [Flammable liquid];Isobutyroyl chloride;Chloro isopropyl ketone;Propanoyl chloride, 2-methyl-;Dimethylacetyl chloride;Isobutyric acid chloride;2-Methylprop-anoyl chloride;α-Methylpropionyl chloride;
- PSA 17.07000
- LogP 1.40780
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphorus trichloride at 45 - 55℃; for 6h; | 99.6% |
| With trichloroacetonitrile; triphenylphosphine In dichloromethane at 30℃; Temperature; Inert atmosphere; | 99.76% |
| Stage #1: isobutyric Acid With phosphorus trichloride at 60℃; for 2h; Stage #2: With hydrogenchloride for 0.3h; Temperature; | 97.5% |
-
-
155879-72-4
2-(5-amino-2-ethoxyphenyl)-pyrido[3,2-d]pyrimidin-4(3H)-one

-
B
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| A 80% B n/a |
-
-
16883-61-7
trimethylsilyl isobutyrate

-
-
4353-77-9
chlorosulfonate de trimethylsilyle

-
A
-
89056-04-2
2-Methyl-2-(trimethylsiloxysulfonyl)-propansaeure-trimethylsilylester

-
B
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane for 12h; Heating; | A 61% B n/a |

-
-
383865-57-4
4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl-amine

-
B
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| A 8% B n/a |

| Conditions | Yield |
|---|---|
| durch Destillation; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; dichloromethane; chlorine |

| Conditions | Yield |
|---|---|
| aluminium trichloride; Pd(Ph3)3(Cl)(COCH3)+AlCl3 In chloroform-d1 at 50℃; for 168h; Product distribution; variation of calyst; |
-
-
35586-36-8
isobutyryl radical

-
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| With tetrachloromethane In hexane at 22.9℃; Rate constant; |
-
-
75-09-2
dichloromethane

-
-
7782-50-5
chlorine

-
-
3619-17-8
isobutyric acid hydrazide

-
-
79-30-1
isobutyryl chloride


-
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| With trichlorophosphate |
-
-
1120-64-5
2-methyl-4,5-dihydro-1,3-oxazole

-
-
75-36-5
acetyl chloride

-
A
-
79-30-1
isobutyryl chloride

-
B
-
13670-39-8
1-(4,5-dihydrooxazol-2-yl)propan-2-one

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran |

-
-
69151-13-9
C3Cl2(C3H7)2

-
-
79-31-2
isobutyric Acid

-
A
-
877675-72-4
2,3-diisopropylcyclopropenone

-
B
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 0.0833333h; Inert atmosphere; | A 66.9 mg B n/a |


| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In dichloromethane at 20℃; Inert atmosphere; | |
| With trichloroisocyanuric acid In dichloromethane at 20℃; Inert atmosphere; |
-
-
707-74-4
(trichloromethyl)mesitylene

-
-
79-31-2
isobutyric Acid

-
A
-
79-30-1
isobutyryl chloride

-
B
-
938-18-1
mesitylene-2-carboxylic acid chloride

| Conditions | Yield |
|---|---|
| With iron(III) chloride at 100℃; for 3h; Inert atmosphere; | A 6.7 kg B 11.5 kg |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide at 0℃; for 4h; | 100% |
| With benzene |
-
-
98-82-8
Isopropylbenzene

-
-
79-30-1
isobutyryl chloride

-
-
72846-62-9
1-(4-isopropylphenyl)-2-methyl-1-propanone

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 20℃; for 2.5h; Inert atmosphere; | 100% |
| With aluminium trichloride at 25℃; Product distribution; | |
| With carbon disulfide; aluminium trichloride | |
| Friedel-Crafts Acylation; | |
| With aluminum (III) chloride In dichloromethane at 0℃; for 2h; Friedel-Crafts Acylation; Sealed tube; Inert atmosphere; |
-
-
92-52-4
biphenyl

-
-
79-30-1
isobutyryl chloride

-
-
6976-20-1
1-([1,1′-biphenyl]-4-yl)-2-methylpropan-1-one

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 0 - 20℃; for 14.5h; | 100% |
| With aluminium trichloride In carbon disulfide Friedel-Crafts acylation; Heating; | 54% |
| With carbon disulfide; aluminium trichloride | |
| With aluminium trichloride In carbon disulfide | |
| With aluminum (III) chloride In 1,2-dichloro-ethane at 5 - 10℃; for 5h; Solvent; Temperature; |
-
-
79-30-1
isobutyryl chloride

-
-
108-46-3
recorcinol

-
-
29048-54-2
1-(2,4-dihydroxyphenyl)-2-methyl-1-propanone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 20℃; | 100% |
| at 90℃; | |
| With aluminium trichloride; nitrobenzene at 40 - 50℃; |
-
-
13059-93-3
monohydroxymethyl-alpha-terthiophene

-
-
79-30-1
isobutyryl chloride

-
-
26905-75-9
5-isobutyryloxymethyl-[2,2';5',2'']terthiophene

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 100% |
-
-
79-30-1
isobutyryl chloride

-
-
151929-68-9
methyl 2,6-anhydro-3-C-methyl-2-thio-β-L-mannopyranoside

-
-
151929-69-0
methyl 2,6-anhydro-4-O-isobutyryl-3-C-methyl-2-thio-β-L-mannopyranoside

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 25℃; for 1.5h; | 100% |
| With pyridine; dmap at 25℃; for 1.5h; | 100% |
-
-
1000334-09-7
N-[2-(6-amino-7-hydroxy-2,3-dihydro-1H-inden-1-yl)ethyl]acetamide hydrochloride

-
-
79-30-1
isobutyryl chloride

-
-
1000334-12-2
N-(3-(2-(acetylamino)ethyl)-4-hydroxy-2,3-dihydro-1H-inden-5-yl)-2-methylpropanamide

| Conditions | Yield |
|---|---|
| With pyridine at 0℃; for 3h; | 100% |
-
-
17422-33-2
6-chloroindole

-
-
79-30-1
isobutyryl chloride

-
-
1002095-56-8
1-(6-chloro-1H-indol-3-yl)-2-methyl-1-propanone

| Conditions | Yield |
|---|---|
| Stage #1: 6-chloroindole With methylmagnesium bromide In diethyl ether for 0.333333h; Stage #2: With zinc(II) chloride In diethyl ether for 0.5h; Stage #3: isobutyryl chloride In diethyl ether Further stages.; | 100% |
| Stage #1: 6-chloroindole; methylmagnesium bromide In diethyl ether for 0.25h; Stage #2: With zinc(II) chloride In diethyl ether for 0.5h; Stage #3: isobutyryl chloride With ammonium chloride more than 3 stages; |
-
-
15297-98-0, 41019-52-7, 41135-46-0
3-Hydroxy-2,2-dimethyl-3,4-dihydro-2H-benzo[h]chromene-5,6-dione

-
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; for 18h; | 100% |
-
-
892388-55-5
(S)-3-[(biphenyl-2-ylmethyl)-amino]-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
79-30-1
isobutyryl chloride

-
-
892388-56-6
tert-butyl (3S)-3-[(biphenyl-2-ylmethyl)(isobutyryl)amino]pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane at 70℃; for 2h; | 100% |
| With triethylamine In 1,4-dioxane at 70℃; for 2h; | |
| With triethylamine In 1,4-dioxane at 20℃; |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; for 2h; | 100% |
-
-
280110-63-6, 782493-60-1
4-(N-hydroxycarbamimidoyl)piperidine-1-carboxylic acid tert-butyl ester

-
-
79-30-1
isobutyryl chloride

| Conditions | Yield |
|---|---|
| With triethylamine; dmap In dichloromethane at 5 - 20℃; for 18.25h; | 100% |
-
-
50541-93-0
4-amino-1-benzylpiperidine

-
-
79-30-1
isobutyryl chloride

-
-
312924-69-9
N-(1-benzylpiperidin-4-yl)isobutyramide

| Conditions | Yield |
|---|---|
| With sodium carbonate In dichloromethane at 20℃; for 2h; | 100% |
| With sodium hydroxide In diethyl ether at 20℃; | 96% |
| With triethylamine In dichloromethane at 20℃; for 1h; | 60% |
| With triethylamine In dichloromethane at 0℃; Inert atmosphere; | |
| With sodium hydroxide In diethyl ether; water at 0 - 20℃; for 18.25h; |
-
-
114878-60-3
1-(2-chloro-4-nitro-phenyl)piperazine

-
-
79-30-1
isobutyryl chloride

-
-
354124-99-5
1-(4-(2-chloro-4-nitrophenyl)piperazin-1-yl)-2-methylpropan-1-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 100% |
-
-
79-30-1
isobutyryl chloride

-
-
103-49-1
dibenzylamine

-
-
6284-09-9
2-methyl-N,N-bis(phenylmethyl)-propionamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 14h; Inert atmosphere; | 100% |
| With triethylamine In diethyl ether at 0 - 20℃; | 92% |
-
-
1092113-85-3
(S)-N'-hydroxy-3-(9-methylthio-6-oxo-2,3,3a,4-tetrahydro-1H,6H-5,8,10,10b-tetraazabenzo[e]azulen-5-yl)benzamidine

-
-
79-30-1
isobutyryl chloride

-
-
1092113-86-4
(S)-5-[3-(5-isopropyl-1,2,4-oxadiazol-3-yl)phenyl]-9-methylthio-1,2,3,3a,4,5-hexahydro-5,8,10,10b-tetraazabenzo[e]azulen-6-one

| Conditions | Yield |
|---|---|
| With pyridine at 90℃; | 100% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0℃; Friedel Crafts Acylation; Inert atmosphere; | 100% |
-
-
876954-00-6
(2S,4R)-4-[2-amino-6-(2-nitrophenoxy)-purin-9-yl]-2-(tert-butoxycarbonylmethoxymethyl)-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
79-30-1
isobutyryl chloride

-
-
876954-02-8
(2S,4R)-2-(tert-butoxycarbonylmethoxymethyl)-4-[2-isobutyrylamino-6-(2-nitrophenoxy)-purin-9-yl]-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; Cooling with ice; | 100% |
-
-
1076199-77-3
racemic 3-(3-N,N'-diisopropylamino-1-phenyl-propyl)-4-hydroxy-benzoic acid

-
-
79-30-1
isobutyryl chloride

-
-
1262778-55-1
2-(3-N,N-diisopropylamine-1-phenylpropyl)-4-carboxyphenol isobutyrate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 25 - 30℃; | 100% |
| With triethylamine In dichloromethane at 25 - 30℃; |
-
-
1292318-07-0
(4-(2-chloropyrimidin-4-yl)aniline)

-
-
79-30-1
isobutyryl chloride

-
-
1292318-08-1
N-[4-(2-chloropyrimidin-4-yl)phenyl]-2-methyl-propanamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 0.5h; | 100% |
| With triethylamine In dichloromethane at 20℃; for 0.5h; | 100% |
-
-
1319068-06-8
[8-(4-fluoro-phenyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[1,5-a]pyrazin-2-yl]-[di-(tert-butoxycarbonyl)]-amine

-
-
79-30-1
isobutyryl chloride

-
-
1319068-09-1
[8-(4-fluoro-phenyl)-7-isobutyryl-5,6,7,8-tetrahydro-[1,2,4]triazolo[1,5-a]pyrazin-2-yl]-[di-(tert-butoxycarbonyl)]-amine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0 - 50℃; for 16h; | 100% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0 - 50℃; for 16h; | 100% |
Isobutyryl Chloride Specification
The Isobutyryl Chloride with CAS registry number of 79-30-1 is also known as Propanoyl chloride, 2-methyl-. The IUPAC name is 2-Methylpropanoyl chloride. It belongs to product categories of Organics; Biochemistry; Nucleosides, Nucleotides & Related Reagents; Protecting Agents for Hydroxyl and Amino Groups; Protecting Agents, Phosphorylating Agents & Condensing Agents; Acid Halides; Carbonyl Compounds; Organic Building Blocks. Its EINECS registry number is 201-194-1. In addition, the formula is C4H7ClO and the molecular weight is 106.55. This chemical is a colorless liquid and should be sealed in a dry place.
Physical properties about Isobutyryl Chloride are: (1)ACD/LogP: 1.34; (2)ACD/LogD (pH 5.5): 1.34; (3)ACD/LogD (pH 7.4): 1.34; (4)ACD/BCF (pH 5.5): 6.1; (5)ACD/BCF (pH 7.4): 6.1; (6)ACD/KOC (pH 5.5): 126.99; (7)ACD/KOC (pH 7.4): 126.99; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 1; (10)Index of Refraction: 1.406; (11)Molar Refractivity: 25.41 cm3; (12)Molar Volume: 103.4 cm3; (13)Surface Tension: 25.3 dyne/cm; (14)Density: 1.03 g/cm3; (15)Flash Point: 1.1 °C; (16)Enthalpy of Vaporization: 33.08 kJ/mol; (17)Boiling Point: 90.9 °C at 760 mmHg; (18)Vapour Pressure: 55.2 mmHg at 25 °C.
Preparation of Isobutyryl Chloride: it is prepared by reaction of isobutyric acid with thionyl chloride. Firstly, isobutyric acid is dropped into thionyl chloride under stirring. Secondly, the reaction mixture is heated to 80 °C for 30 minutes. At last, the mixture is distilled and product is obtained by collecting fraction of 92 °C.
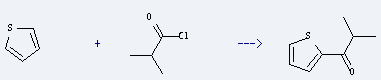
Uses of Isobutyryl Chloride: it is used as intermediates in organic synthesis. It is used to produce 2-methyl-1-thiophen-2-yl-propan-1-one by reaction with thiophene. The reaction needs reagent tin(IV) chloride and the yield is about 75%.
When you are using this chemical, please be cautious about it. As a chemical, it is highly flammable and toxic by inhalation. Besides, it may cause severe burns. During using it, wear suitable protective clothing, gloves and eye/face protection. Keep away from sources of ignition and Do not breathe gas/fumes/vapour/spray. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If accident happens or you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CC(C)C(=O)Cl;
2. InChI: InChI=1S/C4H7ClO/c1-3(2)4(5)6/h3H,1-2H3;
3. InChIKey: DGMOBVGABMBZSB-UHFFFAOYSA-N;
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LCLo | inhalation | 11600mg/m3/6H (11600mg/m3) | LUNGS, THORAX, OR RESPIRATION: "FIBROSIS, FOCAL (PNEUMOCONIOSIS)" SKIN AND APPENDAGES (SKIN): HAIR: OTHER LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | National Technical Information Service. Vol. OTS0555053, |
Related Products
- Isobutyryl Chloride
- Isobutyryl L-Carnitine Chloride
- 793-06-6
- 79307-93-0
- 79-31-2
- 79313-77-2
- 793-19-1
- 79319-85-0
- 79322-83-1
- 79322-97-7
- 79324-50-8
- 79324-77-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View