-
Name
Isophosphamide
- EINECS 223-237-3
- CAS No. 3778-73-2
- Article Data13
- CAS DataBase
- Density 1.33 g/cm3
- Solubility
- Melting Point 48 °C
- Formula C7H15Cl2N2O2P
- Boiling Point 336.1 °C at 760 mmHg
- Molecular Weight 261.088
- Flash Point 157.1 °C
- Transport Information
- Appearance Crystalline Solid
- Safety 26-45
- Risk Codes 25-36
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 2H-1,3,2-Oxazaphosphorine,3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide (8CI);3-(2-Chloroethyl)-2-(2-chloroethylamino)tetrahydro-2H-1,3,2-oxaazaphosphorin2-oxide;A 4942;Asta Z 4942;Cyfos;Holoxan;Holoxan 1000;Ifex;Ifomide;Ifosfamid;Ifosfamide;MJF 9325;Mitoxana;NSC 109724;Naxamide;Z 4942;Ifosfamide;
- PSA 51.38000
- LogP 2.21280
Synthetic route
-
-
29102-47-4
2-aziridino-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin 2-oxide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In chloroform | 78% |
-
-
84681-45-8
(2S)-2-<(chloroacetyl)amino>-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| With B2F6 In tetrahydrofuran for 12h; Ambient temperature; | 28% |

-
A
-
3778-73-2
ilfosfamide

-
B
-
10199-13-0
2-chloro-1,3,5-trimethyl-2-oxo-2λ5-[1,3,5,2]triazaphosphinane-4,6-dione

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In dichloromethane at 0℃; for 1h; | A 14.8% B n/a |
-
-
72578-72-4
3-chloroacetyl-2-(2-chloro-ethylamino)-[1,3,2]oxazaphosphinane (S)-2-oxide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| With diborane In tetrahydrofuran |
-
-
72578-71-3
3-chloroacetyl-2-(2-chloro-ethylamino)-[1,3,2]oxazaphosphinane (R)-2-oxide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| With diborane In tetrahydrofuran |
-
-
81485-04-3
3-(chloroethyl)-2-chlorooxaazaphosphorinane 2-oxide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 94.9 percent / Et3N / CH2Cl2 / 5 h / -15 °C 2: 86.5 percent / CH2Cl2 / 24 h / Ambient temperature 3: 14.8 percent / SO2Cl2 / CH2Cl2 / 1 h / 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1: triethylamine / benzene / 15 h 2: 47 percent / H2 / 10percent Pd/C / ethanol / 96 h / Ambient temperature 3: 50 percent / tetrahydrofuran / 12 h / Ambient temperature 4: 28 percent / B2F6 / tetrahydrofuran / 12 h / Ambient temperature View Scheme |
-
-
195966-73-5
[3-(2-Chloro-ethyl)-2-oxo-2λ5-[1,3,2]oxazaphosphinan-2-yl]-(2-trimethylsilanyloxy-ethyl)-amine

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86.5 percent / CH2Cl2 / 24 h / Ambient temperature 2: 14.8 percent / SO2Cl2 / CH2Cl2 / 1 h / 0 °C View Scheme |
-
-
31190-87-1
3-(1-Ethylenimino)-1-propanol

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 41 percent / triethylamine, phosphoryl chloride / benzene / 4 h 2: triethylamine / benzene / 15 h 3: 47 percent / H2 / 10percent Pd/C / ethanol / 96 h / Ambient temperature 4: 50 percent / tetrahydrofuran / 12 h / Ambient temperature 5: 28 percent / B2F6 / tetrahydrofuran / 12 h / Ambient temperature View Scheme |
-
-
83802-21-5
(+)-2-Dechloroethylifosfamide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 50 percent / tetrahydrofuran / 12 h / Ambient temperature 2: 28 percent / B2F6 / tetrahydrofuran / 12 h / Ambient temperature View Scheme |
-
-
84681-43-6
(1'R,2S)-2-<(1'-methylbenzyl)amino>-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 47 percent / H2 / 10percent Pd/C / ethanol / 96 h / Ambient temperature 2: 50 percent / tetrahydrofuran / 12 h / Ambient temperature 3: 28 percent / B2F6 / tetrahydrofuran / 12 h / Ambient temperature View Scheme |
-
-
36761-83-8
Dechloroethylcyclophosphamide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrahydrofuran 2: B2H6 / tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1: tetrahydrofuran 2: B2H6 / tetrahydrofuran View Scheme |
-
-
72578-61-1
(R)-2-aziridin-1-yl-3-((R)-1-phenyl-ethyl)-[1,3,2]oxazaphosphinane 2-oxide

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: aq. HCl / diethyl ether 2: H2 / Pd-C / ethanol 3: tetrahydrofuran 4: B2H6 / tetrahydrofuran View Scheme | |
| Multi-step reaction with 4 steps 1: aq. HCl / diethyl ether 2: H2 / Pd-C / ethanol 3: tetrahydrofuran 4: B2H6 / tetrahydrofuran View Scheme | |
| Multi-step reaction with 4 steps 1: aq. HCl / diethyl ether 2: H2 / Pd-C / ethanol 3: tetrahydrofuran 4: B2H6 / tetrahydrofuran View Scheme | |
| Multi-step reaction with 4 steps 1: aq. HCl / diethyl ether 2: H2 / Pd-C / ethanol 3: tetrahydrofuran 4: B2H6 / tetrahydrofuran View Scheme |
-
-
72578-68-8
(2-chloro-ethyl)-[(S)-2-oxo-3-((R)-1-phenyl-ethyl)-2λ5-[1,3,2]oxazaphosphinan-2-yl]-amine

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2 / Pd-C / ethanol 2: tetrahydrofuran 3: B2H6 / tetrahydrofuran View Scheme | |
| Multi-step reaction with 3 steps 1: H2 / Pd-C / ethanol 2: tetrahydrofuran 3: B2H6 / tetrahydrofuran View Scheme |
-
-
72578-67-7
(2-chloro-ethyl)-[(R)-2-oxo-3-((R)-1-phenyl-ethyl)-2λ5-[1,3,2]oxazaphosphinan-2-yl]-amine

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2 / Pd-C / ethanol 2: tetrahydrofuran 3: B2H6 / tetrahydrofuran View Scheme | |
| Multi-step reaction with 3 steps 1: H2 / Pd-C / ethanol 2: tetrahydrofuran 3: B2H6 / tetrahydrofuran View Scheme |
-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 57% |
-
-
67-56-1
methanol

-
-
3778-73-2
ilfosfamide

-
-
64858-51-1, 64858-52-2
(+/-)-4-β-methoxyifosfamide

-
-
64858-51-1, 64858-52-2
(+/-)-4-α-methoxyifosfamide

| Conditions | Yield |
|---|---|
| With tetraethylammonium tosylate Electrochemical reaction; 2.2 F/mol electricity, i=15 mA; | A 36% B 48% |


| Conditions | Yield |
|---|---|
| With N,N,N,N-tetraethylammonium tetrafluoroborate In acetonitrile Concentration; Electrochemical reaction; | 41% |

| Conditions | Yield |
|---|---|
| With N,N,N,N-tetraethylammonium tetrafluoroborate In acetonitrile Electrochemical reaction; | 27% |
-
-
5343-92-0
1,2-pentanediol

-
-
3778-73-2
ilfosfamide

-
-
1380481-59-3
1-[3-(2-chloroethyl)-2-(2-chloroethylamino)-2-oxo-2λ5-[1,3,2]oxazaphosphinan-4-yloxy]pentan-2-ol

| Conditions | Yield |
|---|---|
| With N,N,N,N-tetraethylammonium tetrafluoroborate In acetonitrile Electrochemical reaction; | 22% |

| Conditions | Yield |
|---|---|
| With N,N,N,N-tetraethylammonium tetrafluoroborate In acetonitrile Electrochemical reaction; | 19% |
-
-
3778-73-2
ilfosfamide

-
A
-
42436-20-4
rac-4-ketoifosfamide

-
-
39800-28-7, 59884-18-3, 64858-36-2
rac-cis-4-hydroperoxyifosfamide

-
-
39800-28-7, 59884-18-3, 64858-36-2
rac-trans-4-hydroperoxyifosfamide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; ozone In acetone at 0℃; for 4h; | A n/a B 8% C 16% |

-
-
3778-73-2
ilfosfamide

-
-
84681-42-5
(-)-4-ketoifosfamide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iron(II) sulfate In water at 0℃; for 1h; | 5% |
-
-
3778-73-2
ilfosfamide

-
-
72578-74-6
(S)-(-)-2-aziridino-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin 2-oxide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran |
-
-
3778-73-2
ilfosfamide

-
-
72578-73-5
(R)-(+)-2-aziridino-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin 2-oxide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran |
-
-
3778-73-2
ilfosfamide

-
-
64724-15-8
Phosphoric acid mono-{3-[(2-amino-ethyl)-(2-hydroxy-ethyl)-amino]-propyl} ester

| Conditions | Yield |
|---|---|
| In water |
-
-
3778-73-2
ilfosfamide

-
-
29102-47-4
2-aziridino-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin 2-oxide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; aluminum nickel In methanol Product distribution; degradation under various conditions with preparation of nonmutagenic reaction mixtures of products; |
-
-
3778-73-2
ilfosfamide

-
A
-
689-98-5
chloroethylamine

-
B
-
141056-56-6
2-hydroxy-2-oxo-3-(2-chloroethyl)-1,3,2-oxazaphosphorine

-
D
-
241482-17-7
(2-chloro-ethyl)-phosphoramidic acid mono-[3-(2-chloro-ethylamino)-propyl] ester

| Conditions | Yield |
|---|---|
| With sodium phosphate at 20℃; Rate constant; other subst. ifosfamides; var. pH; |

-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; perchloric acid 1.) RT, 24 h, 2.) RT, 24 h; Multistep reaction; |
-
-
3778-73-2
ilfosfamide

-
B
-
241482-17-7
(2-chloro-ethyl)-phosphoramidic acid mono-[3-(2-chloro-ethylamino)-propyl] ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.5h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With ethylenediaminetetraacetic acid; NADPH; supersomes P450 In phosphate buffer at 37℃; for 0.5h; pH=7.4; Enzyme kinetics; Product distribution; |
-
-
3778-73-2
ilfosfamide

-
-
141056-56-6
2-hydroxy-2-oxo-3-(2-chloroethyl)-1,3,2-oxazaphosphorine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. HCl / 0.5 h / Ambient temperature 2: aq. HCl View Scheme |
-
-
3778-73-2
ilfosfamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaH / tetrahydrofuran 2: HI / diethyl ether View Scheme |
-
-
3778-73-2
ilfosfamide

-
-
110971-91-0
N-(2-acetyloxyethyl)-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaH / tetrahydrofuran 2: 43 percent / 1 h / Ambient temperature View Scheme |
Isophosphamide Chemical Properties
IUPAC Name: N,2-bis(2-chloroethyl)-1-oxo-6-oxa-2-aza-1λ5-phosphacyclohexan-1-amine
Molecular Formula:C7H15Cl2N2O2P
Molecular Weight:261.11g/mol
EINECS: 223-237-3
Structure of Isophosphamide (CAS NO.3778-73-2):
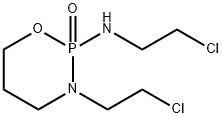
Index of Refraction: 1.505
Molar Refractivity: 58.09 cm3
Molar Volume: 195.6 cm3
Polarizability: 23.03×10-24cm3
Surface Tension: 44.3 dyne/cm
Density: 1.33 g/cm3
Flash Point: 157.1 °C
Enthalpy of Vaporization: 57.92 kJ/mol
Melting Point: 48°C
Boiling Point: 336.1 °C at 760 mmHg
Vapour Pressure: 0.000115 mmHg at 25°C
Product Categories: Anti-Cancer;Pharmaceutical material and intermeidates;Antibiotics;Intermediates & Fine Chemicals;Pharmaceuticals
Canonical SMILES: C1CN(P(=O)(OC1)NCCCl)CCCl
InChI: InChI=1S/C7H15Cl2N2O2P/c8-2-4-10-14(12)11(6-3-9)5-1-7-13-14/h1-7H2,(H,10,12)
InChIKey: HOMGKSMUEGBAAB-UHFFFAOYSA-N
Isophosphamide Uses
Isophosphamide (3778-73-2) can be used as a cytostatic agent, related structurally to cyclophosphamide.
Isophosphamide Toxicity Data With Reference
Organism Test Type Route Reported Dose (Normalized Dose) Effect Source cat LDLo intraperitoneal 100mg/kg (100mg/kg) BEHAVIORAL: ATAXIA
LUNGS, THORAX, OR RESPIRATION: DYSPNEAKiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. dog LDLo intraperitoneal 50mg/kg (50mg/kg) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONKiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. dog LDLo intravenous 66mg/kg (66mg/kg) LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA
KIDNEY, URETER, AND BLADDER: OTHER CHANGES
BLOOD: HEMORRHAGENational Technical Information Service. Vol. PB220-927, guinea pig LDLo intraperitoneal 400mg/kg (400mg/kg) SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE
BEHAVIORAL: ATAXIA
BEHAVIORAL: MUSCLE WEAKNESSKiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. human TDLo intravenous 130mg/kg/13D- (130mg/kg) GASTROINTESTINAL: NAUSEA OR VOMITING
KIDNEY, URETER, AND BLADDER: HEMATURIA
KIDNEY, URETER, AND BLADDER: OTHER CHANGESCancer Research. Vol. 32, Pg. 921, 1972. human TDLo intravenous 1915mg/kg/2W- (1915mg/kg) KIDNEY, URETER, AND BLADDER: HEMATURIA
KIDNEY, URETER, AND BLADDER: "INFLAMMATION, NECROSIS, OR SCARRING OF BLADDER"Lancet. Vol. 2, Pg. 657, 1980. human TDLo intravenous 2298mg/kg/3D- (2298mg/kg) KIDNEY, URETER, AND BLADDER: HEMATURIA
BLOOD: LEUKOPENIAEuropean Journal of Cancer. Vol. 12, Pg. 195, 1976. human TDLo intravenous 2873mg/kg/5D- (2873mg/kg) BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS"
KIDNEY, URETER, AND BLADDER: HEMATURIACancer Research. Vol. 36, Pg. 2945, 1976. human TDLo oral 100mg/kg (100mg/kg) BLOOD: LEUKOPENIA
BLOOD: THROMBOCYTOPENIACancer Chemotherapy Reports, Part 3. Vol. 3, Pg. 33, 1972. human TDLo oral 150mg/kg (150mg/kg) GASTROINTESTINAL: NAUSEA OR VOMITING
KIDNEY, URETER, AND BLADDER: PROTEINURIS
SKIN AND APPENDAGES (SKIN): HAIR: OTHERCancer Research. Vol. 32, Pg. 921, 1972. mouse LD50 intraperitoneal 397mg/kg (397mg/kg) Arzneimittel-Forschung. Drug Research. Vol. 24, Pg. 1149, 1974. mouse LD50 intravenous 338mg/kg (338mg/kg) SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE
LUNGS, THORAX, OR RESPIRATION: DYSPNEA
BEHAVIORAL: ATAXIAKiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. mouse LD50 oral 1005mg/kg (1005mg/kg) SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE
LUNGS, THORAX, OR RESPIRATION: DYSPNEA
BEHAVIORAL: ATAXIAKiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. mouse LD50 subcutaneous 656mg/kg (656mg/kg) SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE
BEHAVIORAL: ATAXIA
LUNGS, THORAX, OR RESPIRATION: DYSPNEAKiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. rabbit LDLo intraperitoneal 200mg/kg (200mg/kg) SENSE ORGANS AND SPECIAL SENSES: MIOSIS (PUPILLARY CONSTRICTION): EYE
BEHAVIORAL: MUSCLE WEAKNESS
LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONKiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. rat LD50 intraperitoneal 140mg/kg (140mg/kg) United States Patent Document. Vol. #3732340, rat LD50 intravenous 190mg/kg (190mg/kg) BLOOD: NORMOCYTIC ANEMIA Kiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. rat LD50 oral 143mg/kg (143mg/kg) BLOOD: NORMOCYTIC ANEMIA Kiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. rat LD50 subcutaneous 160mg/kg (160mg/kg) BLOOD: NORMOCYTIC ANEMIA Kiso to Rinsho. Clinical Report. Vol. 16, Pg. 431, 1982. rat LD50 unreported 325mg/kg (325mg/kg) Arzneimittel-Forschung. Drug Research. Vol. 25, Pg. 1369, 1975. women TDLo intravenous 218mg/kg (218mg/kg) BRAIN AND COVERINGS: DEMYELINATION
BRAIN AND COVERINGS: CHANGES IN SURFACE EEGJournal of Toxicology, Clinical Toxicology. Vol. 27, Pg. 293, 1989.
Isophosphamide Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 , 1987,p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 26 , 1981,p. 237.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . NCI Carcinogenesis Bioassay (ipr); Clear Evidence: mouse, rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-32 ,1977. . EPA Genetic Toxicology Program.
Isophosphamide Safety Profile
Hazard Codes:  T
T
Risk Statements: 25-36
R25 :Toxic if swallowed.
R36:Irritating to eyes.
Safety Statements: 26-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: 3249
RTECS: RP6050000
HazardClass: 6.1(b)
PackingGroup: III
Suspected carcinogen with experimental carcinogenic and neoplastigenic data. A poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Human systemic effects by ingestion and intravenous routes: nausea or vomiting; proteinuria, hematuria, inflammation, necrosis or scarring of the bladder, and other kidney, ureter, or bladder changes; changes in hair covering the skin; leukopenia (decreased white blood cell count), thrombocytopenia (decrease in the number of blood platelets); hallucinations, distorted perceptions; tumorigenic effects (active as an anti-cancer agent). Experimental teratogenic and reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic Cl−, NOx, and POx.
Isophosphamide Specification
Isophosphamide , its cas register number is 3778-73-2. It also can be called 2,3-(N,N(sup 1)-Bis(2-chloroethyl)diamido)-1,3,2-oxazaphosphoridinoxyd ; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)perhydro-2H-1,3,2-oxazaphosphorine oxide ; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide ; ASTA Z 4942 ; Cyfos ; HSDB 7023 ; Holoxan ; I-Phosphamide ; Ifex ; Ifosfamid ; Ifosfamida ; Ifosfamide ; Ifosfamidum ; Ifsofamide ; Iphosphamid ;
Iphosphamide ; Isoendoxan ; Isofosfamide ; Mitoxana ; N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-
amine 2-oxide ; N,N-Bis(beta-chloroethyl)-amino-N',O-propylene-phosphoric acid ester diamide ; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid ; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid [German] ; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid diamide ; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid ester diamide ; Naxamide ; UNII-UM20QQM95Y .
Isophosphamide (CAS NO.3778-73-2) is a crystalline solid.
Related Products
- Isophosphamide
- 3778-76-5
- 37787-90-9
- 37789-75-6
- 3779-03-1
- 3779-27-9
- 3779-29-1
- 377-93-5
- 37793-53-6
- 37794-19-7
- 3779-42-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View