-
Name
L-Alaninamide hydrochloride
- EINECS 608-843-1
- CAS No. 33208-99-0
- Article Data6
- CAS DataBase
- Density
- Solubility
- Melting Point 212-217 °C
- Formula C3H8N2O.HCl
- Boiling Point 247.4 °C at 760 mmHg
- Molecular Weight 124.57
- Flash Point 103.4 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms L-Alanine amide hydrochloride;H-ALA-NH2.HCL;Propanamide, 2-amino-, monohydrochloride, (2S)-(9CI);Propanamide, 2-amino-, monohydrochloride, (S)-;(2S)-2-Aminopropanamide hydrochloride;Alanine amide hydrochloride;
- PSA 69.11000
- LogP 1.02150
Synthetic route
-
-
302-72-7
rac-Ala-OH

-
A
-
33208-99-0
(S)-alaninamide hydrochloride

-
B
-
71810-97-4
D-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| With laccase; ammonia In water at 38 - 40℃; Temperature; Enzymatic reaction; | A 92% B n/a |
-
-
78981-25-6, 81587-17-9, 85642-13-3
tert-butyl (1S)-2-amino-1-methyl-2-oxoethylcarbamate

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| With acetyl chloride In ethanol; ethyl acetate for 1h; | 80% |
| With hydrogenchloride In 1,4-dioxane at 80℃; for 4h; |
-
-
2491-20-5
L-alanine methyl ester hydrochloride

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| With ammonia In methanol for 72h; Ambient temperature; | |
| Stage #1: L-alanine methyl ester hydrochloride With ammonia at 20℃; for 20h; Sealed tube; Large scale; Stage #2: With hydrogenchloride In water pH=1.55; pH-value; Large scale; | 85 g |
-
-
16251-77-7
3-Phenylbutyraldehyde

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
1084907-53-8
(5S)-5-methyl-2-(2-phenylpropyl)imidazolidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; triethylamine In ethanol at 60℃; for 24h; | 100% |
| With potassium carbonate; triethylamine In ethanol at 60℃; for 24h; Inert atmosphere; | 100% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| With N-ethylmorpholine; In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
130981-51-0
(2S,2'S)-4,4'-disulfanediylbis(2-(tert-butoxycarbonyl)amino)butanoic acid

-
-
569341-03-3
(Boc-Hcy-Ala-NH2)2

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 5h; | 99% |
-
-
7536-58-5
BOC-L-aspartic acid 4-benzyl ester

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
302911-12-2
BOC-Asp(OBn)-Ala-NH2

| Conditions | Yield |
|---|---|
| Stage #1: BOC-L-aspartic acid 4-benzyl ester With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran; N,N-dimethyl-formamide at -40 - 0℃; for 1h; Stage #2: (S)-alaninamide hydrochloride With 4-methyl-morpholine In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 24h; | 99% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 0.5h; Stage #2: 3-((quinoline-8-oxy)methyl)benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 96.08% |
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 1h; Stage #2: 3-((quinoline-8-oxy)methyl)benzaldehyde In methanol for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 96% |
-
-
124980-31-0
benzyloxycarbonyl-L-leucine benzyl ester

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
62074-75-3
Cbz-Leu-Ala-NH2

| Conditions | Yield |
|---|---|
| With Alcalase In tert-Amyl alcohol at 5℃; for 96h; Condensation; | 96% |
-
-
2389-46-0
N-α-tert-butyloxycarbonyl-N-ε-benzyloxycarbonyl-lysine p-nitrophenyl ester

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
163848-14-4
Nα-BOC-Nε-CBZ-L-lysyl-L-alanine amide

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate; N,N-dimethyl-formamide | 96% |
-
-
27746-99-2
2,2,2-trifluoroethyl chloroformate

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
951242-82-3
N-(2,2,2-trifluoroethoxycarbonyl)-L-alaninamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; ethyl acetate at 20℃; for 2.5h; | 95% |
-
-
53985-49-2
3-chloro-5-(trifluoromethyl)benzoic acid

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In ethyl acetate; N,N-dimethyl-formamide for 0.5h; | 95% |
-
-
327-92-4
1,5-difluoro-2,4-dinitrobenzene

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With magnesium sulfate; sodium hydroxide In water; acetone at 20℃; for 4h; Stage #2: 1,5-difluoro-2,4-dinitrobenzene In water; acetone at 1℃; for 20h; | 93% |
| Stage #1: (S)-alaninamide hydrochloride With magnesium sulfate; sodium hydroxide In acetone at 20℃; for 3h; Stage #2: 1,5-difluoro-2,4-dinitrobenzene In acetone for 0.5h; | 63% |
-
-
6953-22-6
5-benzyloxyindole-3-carboxaldehyde

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With sodium cyanoborohydride In methanol at 20℃; for 0.25h; Molecular sieve; Inert atmosphere; Stage #2: 5-benzyloxyindole-3-carboxaldehyde In methanol at 20 - 35℃; for 4h; Inert atmosphere; | 92.1% |

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; Stage #2: 4-((2-fluorobenzyl)oxy)benzaldehyde In methanol at 25℃; for 3.33333h; Stage #3: With sodium hydroxide; sodium tetrahydroborate In methanol; water at 8℃; for 4h; Product distribution / selectivity; | 92% |
| Stage #1: 4-((2-fluorobenzyl)oxy)benzaldehyde; (S)-alaninamide hydrochloride With triethylamine In methanol at 20 - 25℃; for 1h; Stage #2: With hydrogen; 5 % platinum on carbon In methanol at 20 - 35℃; under 3750.38 Torr; for 5h; Product distribution / selectivity; | 85% |
| Stage #1: 4-((2-fluorobenzyl)oxy)benzaldehyde; (S)-alaninamide hydrochloride With triethylamine In methanol at 20 - 25℃; for 1h; Stage #2: With hydrogen; palladium 10% on activated carbon In methanol at 20 - 35℃; under 3750.38 Torr; for 5h; Product distribution / selectivity; | 70% |
-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
497262-13-2
N,N'-disuccinimidyl-2,2'-dithiosalicylate

-
-
449775-52-4
N,N'-(2,2'-dithiobisbenzoyl)-bis-L-alaninamide

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; | 92% |
| With triethylamine In N,N-dimethyl-formamide at 25℃; |
-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
96561-04-5, 16965-06-3
(S)-6-trifluoroacetylamino-2-(tert-butoxycarbonylamino)-hexanoic acid

-
-
1382351-57-6
Boc-L-Lys(COCF3)-L-Ala-NH2

| Conditions | Yield |
|---|---|
| With cyano-hydroxyimino-acetic acid 2,2-dimethyl-[1,3]dioxolan-4-ylmethyl ester; sodium hydrogencarbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In water at 20℃; for 2h; optical yield given as %de; | 92% |

| Conditions | Yield |
|---|---|
| With 1-butyl-2,3-dimethylimidazolium tetrafluoroborate; triethylamine at 60 - 70℃; for 6h; Reagent/catalyst; | 92% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 0.5h; Stage #2: 4-((2,3-dihydrobenzo[b]thiophen-7-oxy)methyl)benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 91.67% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 1h; Stage #2: 3-((benzofuran-4-oxy)methyl) benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 91.18% |
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 1h; Stage #2: 3-((benzofuran-4-oxy)methyl) benzaldehyde In methanol for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 91% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; δ-chymotrypsin In hydrogenchloride for 168h; Ambient temperature; | 91% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 1h; Stage #2: 4-((benzofuran-4-oxy)methyl)benzaldehyde In methanol for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 91% |
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 1h; Stage #2: 4-((benzofuran-4-oxy)methyl)benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 55.91% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
78981-25-6, 81587-17-9, 85642-13-3
tert-butyl (1S)-2-amino-1-methyl-2-oxoethylcarbamate

| Conditions | Yield |
|---|---|
| With triethylamine In methanol for 12h; | 90% |
| With triethylamine In dichloromethane for 3h; | 74% |
| With triethylamine In tetrahydrofuran; water at 20℃; for 16h; | 73% |
| With triethylamine In tetrahydrofuran; water at 20℃; for 16h; | 73% |
| With sodium carbonate In 1,4-dioxane; water for 15h; |
-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
97985-98-3
p-methoxybenzyloxycarbonyl-Phe-OMe

-
-
145126-20-1
Moz-Phe-Ala-NH2

| Conditions | Yield |
|---|---|
| With Alcalase In tert-Amyl alcohol at 5℃; for 48h; Condensation; | 90% |
| With triethylamine In tert-butyl alcohol at 35℃; industrial alkaline protease alcalase, 3 to 4 h; | 86% |
-
-
66742-57-2
4-(3-fluoro-benzyloxy)-benzaldehyde

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
133865-89-1
safinamide

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 30℃; for 0.166667h; Stage #2: 4-(3-fluoro-benzyloxy)-benzaldehyde In methanol at 20℃; for 3.5h; Stage #3: With sodium tetrahydroborate In methanol at 5℃; for 2h; Product distribution / selectivity; | 90% |
| Stage #1: 4-(3-fluoro-benzyloxy)-benzaldehyde; (S)-alaninamide hydrochloride With triethylamine In methanol at 20 - 25℃; for 1h; Stage #2: With hydrogen; 5 % platinum on carbon In methanol at 20 - 35℃; under 750.075 - 3750.38 Torr; for 5h; Product distribution / selectivity; | 90% |
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 30℃; for 0.166667h; Stage #2: 4-(3-fluoro-benzyloxy)-benzaldehyde In methanol at 30℃; for 3.5h; Stage #3: With sodium hydroxide; sodium tetrahydroborate In methanol; water at 5℃; for 2h; Product distribution / selectivity; | 88.5% |
-
-
70627-20-2
4-((2-fluorobenzyl)oxy)benzaldehyde

-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
1000370-32-0
(S)-2-[4-(2-fluorobenzyloxy)benzylideneamino]propanamide

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 1h; | 90% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 0.5h; Stage #2: 3-((2,3-dihydrobenzo[b][1,4]dioxin-5-oxy)methyl)benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 88.83% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 0.5h; Stage #2: 3-((2,3-dihydrobenzo[b]thiophen-7-oxy)methyl)benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 88.65% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 0.5h; Stage #2: 4-((2,3-dihydrobenzofuran-5-oxy)methyl)benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 88.31% |

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 0.5h; Stage #2: 4-((1H-benzo[d]imidazole-4-oxy)methyl)benzaldehyde In methanol at 20℃; for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 88.06% |
| Stage #1: (S)-alaninamide hydrochloride With triethylamine In methanol at 20℃; for 1h; Stage #2: 4-((1H-benzo[d]imidazole-4-oxy)methyl)benzaldehyde In methanol for 2h; Stage #3: With potassium borohydride In methanol for 3h; Reflux; | 88% |
-
-
33208-99-0
(S)-alaninamide hydrochloride

-
-
60379-01-3
N-(benzyloxycarbonyl)-L-phenylalanine benzyl ester

-
-
65118-54-9
N-(benzyloxycarbonyl)phenylalanyl-alanine amide

| Conditions | Yield |
|---|---|
| With S166C-SCH2CH2NH3+; triethylamine; calcium chloride In water; N,N-dimethyl-formamide Ambient temperature; | 88% |
| With triethylamine; wild-type serine protease subtilisin Bacillus lentus In water; N,N-dimethyl-formamide at 20℃; for 24h; pH=5.8; | 57% |
-
-
7622-21-1
benzyl-(S)-(-)-2-hydroxy-3-phenylpropionate

-
-
33208-99-0
(S)-alaninamide hydrochloride

| Conditions | Yield |
|---|---|
| With subtilisin bacillus lentus S166C-S-CH2C6F5 mutant enzyme; triethylamine In water; N,N-dimethyl-formamide at 20℃; for 48h; | 88% |
L-Alaninamide hydrochloride Chemical Properties
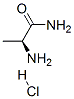
IUPAC Name:(2S)-2-aminopropanamide hydrochloride
Molecular Formula:C3H9ClN2O
Molecular Weight:124.569360 g/mol
Appearance:white to light yellow crystal powder
Melting Point:212-217 °C
alpha:11 °(c=1, MeOH)
storage temp.:2-8 °C
Synonyms of L-Alaninamide hydrochloride(33208-99-0):
(S)-2-AMINOPROPIONAMIDE HYDROCHLORIDE;L-ALANAMINE HCL;(L)-ALANINAMIDE HCL;L-ALANINAMIDE HYDROCHLORIDE;L-ALANINE AMIDE HYDROCHLORIDE;H-L-ALA-NH2 HCL;H-ALA-NH2 HCL;H-ALA-NH2 HYDROCHLORIDE
Categories of L-Alaninamide hydrochloride(33208-99-0):
chiral;Amino Acids;Alanine [Ala, A];Amino hydrochloride;Alanine;Amino Acid Derivatives;Peptide Synthesis
L-Alaninamide hydrochloride Uses
L-Alaninamide hydrochloride Safety Profile
Hazard Codes:C
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View