-
Name
L-Asparagine
- EINECS 200-735-9
- CAS No. 70-47-3
- Article Data80
- CAS DataBase
- Density 1.405 g/cm3
- Solubility Soluble in acid and alkali solution; Slightly soluble in water; Hardly soluble in ethanol, ethyl ether, methanol and benzene
- Melting Point 235 °C (dec.)(lit.)
- Formula C4H8N2O3
- Boiling Point 438.029 °C at 760 mmHg
- Molecular Weight 132.119
- Flash Point 218.712 °C
- Transport Information
- Appearance White crystalline powder
- Safety 24/25-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Asparagine,L- (8CI);(-)-Asparagine;(S)-2,4-Diamino-4-oxobutanoic acid;(S)-Asparagine;Agedoite;Altheine;Asparagine;Asparagine acid;Asparamide;Aspartamic acid;Aspartic acid amide;Aspartic acid b-amide;Butanoicacid, 2,4-diamino-4-oxo-, (S)-;Crystal VI;L-2,4-Diamino-4-oxobutanoic acid;L-Aspartamine;L-b-Asparagine;NSC82391;a-Aminosuccinamicacid;
- PSA 106.41000
- LogP -0.32570
Synthetic route
-
-
111-26-2
hexan-1-amine

-
-
78641-70-0
(S)-2-(3,5-Dinitro-4-oxo-4H-pyridin-1-yl)-succinamic acid

-
A
-
70-47-3
L-asparagine

-
B
-
74197-48-1
1-hexyl-3,5-dinitro-4-pyridone

| Conditions | Yield |
|---|---|
| In pyridine Product distribution; | A 96.2% B n/a |

-
-
71989-16-7
Fmoc-Asn-OH

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide at 50℃; for 20h; | 90% |

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With ruthenium nanoparticles dispersed in a polyvinylpyrrolidone matrix; amberlyst A-21 In methanol; dichloromethane | 90% |
-
-
16856-13-6
(+)-β-methyl-L-aspartate hydrochloride

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With ammonia In tetrahydrofuran; methanol; diethyl ether at 25℃; for 96h; | 77% |
| With ammonium hydroxide at 30℃; under 750.075 Torr; for 9h; Reagent/catalyst; Temperature; Pressure; | 75% |
-
-
97347-33-6
Boc-Asn(Boc)-ONBzl

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol Ambient temperature; | 73% |

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 70℃; for 20h; | 72% |

| Conditions | Yield |
|---|---|
| With ethanol; ammonia at 100℃; das beim Verdunsten der inaktiven Loesung gleicher Teile von d- und l-Asparagin sich ausscheidende Krystallgemenge der aktiven Asparagine laesst sich mechanisch Auslesen trennen; d-asparagine; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid | |
| With palladium Hydrogenation; | |
| With ethylenediaminetetraacetic acid; phenylmethylsulphonyl fluoride; water In aq. phosphate buffer at 30℃; Kinetics; Reagent/catalyst; Microbiological reaction; Enzymatic reaction; |
-
-
42406-52-0
N2,N2-phthaloyl-L-asparagine

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With water; hydrazine hydrate |
-
-
98115-12-9
Nα,Nca-di-tert-butyloxycarbonylasparagine

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With hydrogen bromide In acetic acid |
-
-
101301-51-3
His-Ser-Asn-Gly

-
A
-
56-45-1
L-serin

-
B
-
70-47-3
L-asparagine

-
C
-
56-40-6
glycine

-
D
-
71-00-1
L-histidine

| Conditions | Yield |
|---|---|
| With Tris-HCl buffer In water at 37℃; Product distribution; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium phosphate buffer; porcine kidney acylase I at 40℃; relative initial rate of hydrolysis; |

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With borax; sodium dihydrogenphosphate at 26℃; Equilibrium constant; |

-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In acetic acid at 55℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| at 105 - 110℃; |


-
-
98490-54-1
optically inactive (3,6-dioxo-piperazine-2,5-diyl)-di-acetic acid diamide

-
A
-
2058-58-4
(R)-Asparagine

-
B
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| at 100℃; inactive 3.6-dioxo-piperazine-diacetic acid-(2.5)-diamide; |


-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With methanol; palladium; acetic acid durch Hydrogenolyse; |

| Conditions | Yield |
|---|---|
| -diamide; |

| Conditions | Yield |
|---|---|
| With ammonia; water at 100℃; das beim Verdunsten der inaktiven Loesung gleicher Teile von d- und l-Asparagin sich ausscheidende Krystallgemenge der aktiven Asparagine laesst sich mechanisch Auslesen trennen; d-asparagine; |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In d7-N,N-dimethylformamide; water-d2 at 20℃; for 3h; | 100% |
| With ethanol; sodium hydrogencarbonate |
-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| In water pH=2 - 2.33; Inert atmosphere; Cooling with ice; | 100% |

| Conditions | Yield |
|---|---|
| With N-ethylmorpholine;; toluene-4-sulfonic acid In methanol complex, amino-acid, sulphonic acid, dimethyl sulphite in methanol was esterified at 70 degree.C for 3 h, then N-ethylmorpholine was added; dild. with water, chromd. on Amberlite IRC 50 column (Na-form), evapn. to dryness, taken up in MeOH, filtration, evapn.; | 99% |

-
-
70-47-3
L-asparagine

-
-
630-19-3
pivalaldehyde

-
-
131791-76-9
cis-2-tert-butyl-6(S)-potassium carboxylate-perhydropyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 98% |
| With potassium hydroxide Multistep reaction; | |
| With potassium hydroxide 1.) 60 deg C, overnight, 0.1 torr, 2.) methanol, reflux; Multistep reaction; |
-
-
70-47-3
L-asparagine

-
-
16624-64-9
α-carbobenzoxy-L-tryptophan p-nitrophenyl ester

-
-
1105050-59-6
N-benzyloxycarbonyl-L-tryptophan-L-asparagine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; water at 20℃; | 98% |
-
-
70-47-3
L-asparagine

| Conditions | Yield |
|---|---|
| With pyridine In water at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| In water | 97% |
-
-
70-47-3
L-asparagine

-
-
186371-01-7
2,4-dihydroxybenzoic acid N-hydroxysuccinimidyl ester

| Conditions | Yield |
|---|---|
| With TEA In N,N-dimethyl-formamide for 12h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In water Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| In water at 3℃; for 48h; Darkness; | 95% |

| Conditions | Yield |
|---|---|
| In water pH=3.38 - 3.86; Cooling with ice; | 95% |
-
-
70-47-3
L-asparagine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
36212-66-5
N2-(toluene-4-sulfonyl)-L-asparagine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane for 2h; Ambient temperature; | 94% |
| With triethylamine In tetrahydrofuran; water for 2h; | 92% |
| With sodium hydroxide In 1,4-dioxane a) 0 deg C, 1 h, b) RT, 3 h; | 91% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane at 0℃; for 0.5h; Condensation; | 93% |
| With diethyl ether | |
| Stage #1: L-asparagine; benzenesulfonyl chloride With sodium hydroxide In 1,4-dioxane; water at 0℃; for 3h; Stage #2: With hydrogenchloride; water pH=3; |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 55℃; for 6h; | 92% |
-
-
70-47-3
L-asparagine

-
-
77-98-5
tetraethylammonium hydroxide

-
-
1248586-09-5
tetraethylammonium L-asparaginate

| Conditions | Yield |
|---|---|
| In water at 20℃; for 2h; | 92% |
| In water at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 70℃; for 2h; pH=8.5 - 9; | 92% |
-
-
70-47-3
L-asparagine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
7536-55-2
N-tert-butyloxycarbonylasparagine

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 20℃; | 91% |
| Stage #1: L-asparagine; di-tert-butyl dicarbonate With sodium carbonate In 1,4-dioxane; water at 20℃; Stage #2: With hydrogenchloride In water pH=2; | 91% |
| With sodium carbonate In 1,4-dioxane; water at 25℃; Acylation; | 88% |
-
-
70-47-3
L-asparagine

-
-
103-72-0
phenyl isothiocyanate

-
-
29588-03-2
2-(5-oxo-1-phenyl-2-thioxoimidazolidin-4-yl)acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide at 20℃; for 5h; | 91% |
-
-
70-47-3
L-asparagine

-
-
197244-91-0
1,1-dioxobenzo[b]thiophen-2-ylmethyl N-succimidyl carbonate

-
-
197245-31-1
Bsmoc-Asn-OH

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetone Ambient temperature; | 90.4% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 20℃; for 0.5h; pH=8.5; | 90.4% |

| Conditions | Yield |
|---|---|
| In methanol; benzene byproducts: water; N2-atmosphere; dropwise addn. of Bu2SnO (in hot C6H6/MeOH=3:1 v/v) to 2 equiv. aminoacid (in hot MeOH), stirring and refluxing for 30 min, then refluxing for 9-10 h with azeotropic distn. off of water; solvent removal (reduced pressure), trituration with petroleum ether, recrystn. (MeOH and petroleum ether); elem. anal.; | 90% |
-
-
70-47-3
L-asparagine

-
-
144481-14-1
Carbonic acid 2-(2,4-dinitro-phenyl)-ethyl ester 4-nitro-phenyl ester

-
-
144481-19-6
(S)-2-[2-(2,4-Dinitro-phenyl)-ethoxycarbonylamino]-succinamic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water | 89% |
-
-
70-47-3
L-asparagine

-
-
36983-58-1
L-Aspol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ruthenium-carbon composite; hydrogen at -5 - 30℃; for 14h; | 88.3% |
| With hydrogenchloride; ruthenium-carbon composite; hydrogen In water at -5 - 30℃; for 9h; | 86.2% |
| With hydrogenchloride; ruthenium-carbon composite; hydrogen In water at 20℃; for 10h; | 82.77% |
-
-
70-47-3
L-asparagine

-
-
13248-54-9
cyclohexyl chloroformate

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 3h; Ambient temperature; | 88% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 20℃; for 2h; | 88% |
-
-
70-47-3
L-asparagine

-
-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide

-
-
71989-16-7
Fmoc-Asn-OH

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 0 - 20℃; | 87% |
| Stage #1: L-asparagine With N-cyclohexyl-cyclohexanamine In acetone at 20℃; Stage #2: N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide With sodium carbonate In water; acetonitrile at 0 - 20℃; pH=8; Stage #3: With potassium hydrogensulfate In water; acetonitrile pH=2 - 3; | 59% |
-
-
70-47-3
L-asparagine

-
-
136465-99-1
2,5-dioxopyrrolidin-1-ylquinoline-2-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water; acetone at 20℃; for 12h; | 87% |
L-Asparagine History
L-Asparagine was first isolated in 1806, under a crystalline form, by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant) from asparagus juice, in which it is abundant -- hence the name they chose for that new matter -- becoming the first amino acid to be isolated. The characteristic smell observed in the urine of individuals after their consumption of asparagus is attributed to various metabolic byproducts of asparagine.
A few years later, in 1809, Pierre Jean Robiquet again identified, this time from liquorice root, a substance with properties he qualified as very similar to those of asparagine, that Plisson in 1828 will confirm effectively as asparagine itself .
L-Asparagine Specification
The L-Asparagine, with the CAS registry number 70-47-3, is also known as L-2,4-Diamino-4-oxobutanoic acid. It belongs to the product categories of Amino Acids Derivatives; Amino Acids and Derivatives; Amino Acids; Nutritional Supplements; Amino Acids. Its EINECS number is 200-735-9. This chemical's molecular formula is C4H8N2O3 and molecular weight is 132.12. What's more, its systematic name is L-Asparagine. This chemical is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is a non-essential amino acid that is involved in the metabolic control of cell functions in nerve and brain tissue. It is biosynthesized from aspartic acid and ammonia by asparagine synthetase. This chemical should be stored in an airtight container that is away from light.
Physical properties of L-Asparagine are: (1)ACD/LogP: -1.88; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): -4.37; (4)ACD/LogD (pH 7.4): -4.44; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 5; (10)#H bond donors: 5; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 106.41 Å2; (13)Index of Refraction: 1.533; (14)Molar Refractivity: 29.204 cm3; (15)Molar Volume: 94.039 cm3; (16)Polarizability: 11.577×10-24cm3; (17)Surface Tension: 71.7 dyne/cm; (18)Density: 1.405 g/cm3; (19)Flash Point: 218.712 °C; (20)Enthalpy of Vaporization: 76.174 kJ/mol; (21)Boiling Point: 438.029 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by Boc-Asn(Boc)-ONBzl at the ambient temperature. This reaction will need reagent H2 and solvent ethanol. This reaction will also need catalyst Pd-C (10percent). The yield is about 73%.
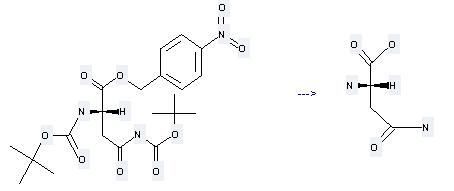
Uses of L-Asparagine: it can be used to produce N2-[(tert-butoxy)carbonyl]-L-asparagine at the temperature of 25 °C. It will need reagent Na2CO3 and solvents dioxane, H2O. The yield is about 88%.
![L-Asparagine can be used to produce N2-[(tert-butoxy)carbonyl]-L-asparagine at the temperature of 25 °C](/UserFilesUpload/Uses of L-Asparagine.jpeg)
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. It is harmful by inhalation, in contact with skin and if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: C([C@@H](C(=O)O)N)C(=O)N
(2)Std. InChI: InChI=1S/C4H8N2O3/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H2,6,7)(H,8,9)/t2-/m0/s1
(3)Std. InChIKey: DCXYFEDJOCDNAF-REOHCLBHSA-N
Related Products
- L-Asparagine
- L-Asparagine methyl ester hydrochloride
- L-Asparagine sulphate
- L-Asparagine tert-butyl ester
- L-Asparagine, glycyl-
- L-Asparagine, N<sup>2</sup>-(diphenylphosphinothioyl)-
- L-Asparagine, N-methyl-
- L-Asparagine,L-methionyl-
- L-Asparagine,N-acetyl-L-alpha-aspartyl-L-alpha-glutamyl-L-valyl-N-(4-nitrophenyl)-(9CI)
- 70476-82-3
- 70476-91-4
- 70477-26-8
- 70477-64-4
- 7047-84-9
- 70478-63-6
- 7048-02-4
- 7048-04-6
- 70482-71-2
- 704-83-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View