-
Name
Malonamide
- EINECS 203-553-8
- CAS No. 108-13-4
- Article Data54
- CAS DataBase
- Density 1.281 g/cm3
- Solubility 180 g/L (20 °C) in water
- Melting Point 172-175 °C(lit.)
- Formula C3H6N2O2
- Boiling Point 460.9 °C at 760 mmHg
- Molecular Weight 102.093
- Flash Point 232.5 °C
- Transport Information
- Appearance white crystalline powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Malonamide(6CI,8CI);Malondiamide;Malonic acid diamide;Malonic diamide;Malonodiamide;NSC 2134;Propanediamide;
- PSA 86.18000
- LogP -0.25230
Synthetic route

| Conditions | Yield |
|---|---|
| With Acetaldehyde oxime In methanol at 65℃; for 4h; | 98% |
| With manganese(IV) oxide; water In isopropyl alcohol at 100℃; under 5171.62 Torr; for 0.5h; | 98% |
| Stage #1: malononitrile With acetamide; acetic acid at 50℃; under 760.051 Torr; for 0.5h; Stage #2: With tetrakis(acetonitrile)palladium(II) tetrafluoroborate at 50℃; under 1 - 3 Torr; for 2h; | 43% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide | 91% |
| With Amberlyst A-26 (OH- form); dihydrogen peroxide In methanol at 20℃; for 4h; Hydrolysis; | 85% |
| With urea-hydrogen peroxide; potassium carbonate In water; acetone for 1.25h; Ambient temperature; |
-
-
19411-83-7
2-benzylidenepropanediamide

-
-
41867-20-3
ethyl (E)-3-aminobut-2-enoate

-
A
-
108-13-4
malonodiamide

-
B
-
1165-06-6
Diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate

-
-
89434-90-2
3-carbamoyl-4-phenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydropyridin-2-one

| Conditions | Yield |
|---|---|
| In ethanol; acetic acid for 1h; Heating; | A 34% B 27% C 13% |


| Conditions | Yield |
|---|---|
| With diethyl ether; ammonia | |
| With ammonia at 0℃; | |
| With ammonia In diethyl ether temperatures below 0°C;; |

| Conditions | Yield |
|---|---|
| With ammonia; ammonium chloride at 0℃; |

| Conditions | Yield |
|---|---|
| With ammonia | |
| With ammonia In ethanol for 24h; Ambient temperature; |
-
-
39000-70-9
methanetetracarboxylic acid tetraethyl ester

-
-
108-13-4
malonodiamide

| Conditions | Yield |
|---|---|
| With ammonia |
-
-
49597-05-9
propene-1,1,3,3-tetracarboxylic acid tetraethyl ester

-
A
-
108-13-4
malonodiamide

| Conditions | Yield |
|---|---|
| With ammonium hydroxide 14-taegiges Stehen; |

| Conditions | Yield |
|---|---|
| at 150 - 160℃; |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide | |
| With ammonia at 25℃; | |
| With ammonia; ammonium chloride at 0℃; | |
| With ammonium hydroxide | |
| With hydrazine hydrate |

| Conditions | Yield |
|---|---|
| With water Irradiation; |

-
-
108-13-4
malonodiamide

| Conditions | Yield |
|---|---|
| With perchloric acid In water at 25℃; Equilibrium constant; |
-
-
855654-96-5
4-hydroxy-6-methoxy-2-oxo-2H-pyran-3-carboxylic acid methyl ester

-
-
108-13-4
malonodiamide
-
-
897-38-1
1,3-Dihydro-3-oxo-2-isobenzofuranylidenmalonsaeurediethylester

-
-
7664-41-7
ammonia

-
A
-
108-13-4
malonodiamide

-
B
-
88-96-0
phthalamide


| Conditions | Yield |
|---|---|
| at 0℃; Beschleunigung der Reaktion durch verschiedene Ammoniumsalze; Geschwindigkeit; | |
| at -33℃; Beschleunigung der Reaktion durch verschiedene Ammoniumsalze; Geschwindigkeit; |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
108-13-4
malonodiamide
-
-
64-17-5
ethanol

-
-
7664-41-7
ammonia

-
-
105-53-3
diethyl malonate

-
A
-
141-82-2
malonic acid

-
B
-
108-13-4
malonodiamide

-
C
-
7597-56-0
ethyl malonamate

-
-
64-17-5
ethanol

-
-
7664-41-7
ammonia

-
-
7732-18-5
water

-
-
105-53-3
diethyl malonate

-
A
-
141-82-2
malonic acid

-
B
-
108-13-4
malonodiamide

-
C
-
7597-56-0
ethyl malonamate

| Conditions | Yield |
|---|---|
| Kinetics; |



| Conditions | Yield |
|---|---|
| at 0℃; Geschwindigkeit; |

| Conditions | Yield |
|---|---|
| at 0℃; Geschwindigkeit; |


| Conditions | Yield |
|---|---|
| at 25℃; bei 60grad rasch; |


| Conditions | Yield |
|---|---|
| at 25℃; oberhalb 60grad rasch; |

-
-
49597-05-9
propene-1,1,3,3-tetracarboxylic acid tetraethyl ester

-
A
-
108-13-4
malonodiamide


| Conditions | Yield |
|---|---|
| With Lawessons reagent In toluene at 100℃; for 4h; | 100% |
| With Lawessons reagent In tetrahydrofuran for 10h; Heating; | 47% |
| With tetraphosphorus decasulfide In 1,4-dioxane for 2h; Heating; | 21% |
| With tetraphosphorus decasulfide In 1,4-dioxane for 1.5h; Heating; | |
| With Lawessons reagent In toluene for 3h; Heating; Yield given; |
-
-
7521-41-7
2-Aminonicotinaldehyde

-
-
108-13-4
malonodiamide

-
-
15935-96-3
2-Amino-1,8-naphthyridine-3-carboxamide

| Conditions | Yield |
|---|---|
| With piperidine In methanol for 0.0333333h; Friedlander condensation; microwave irradiation; | 100% |
-
-
13598-36-2
phosphonic Acid

-
-
108-13-4
malonodiamide

| Conditions | Yield |
|---|---|
| In water pptn. from stoich. mixt. of U-salt, phosphorous acid and amide solns. (c(DAMA) 0.1 M); X-ray diffraction; elem. anal.; | 99% |


| Conditions | Yield |
|---|---|
| With boron trifluoride; tetra(n-butyl)ammonium hydrogensulfate at 80℃; for 15h; Temperature; Reagent/catalyst; Large scale; | 98.8% |
-
-
108-13-4
malonodiamide

-
-
30989-14-1
dichloro-malonic acid diamide

| Conditions | Yield |
|---|---|
| With lead(IV) acetate; aluminium trichloride In acetonitrile for 0.916667h; Heating; | 95% |
| With chlorine | |
| With water; chlorine | |
| With chloroform; Iodine monochloride | |
| With sulfuryl dichloride |
-
-
108-13-4
malonodiamide

-
-
71721-66-9
hydroxyimino-malonic acid diamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 1-butyl-3-methylimidazolium nitrite; water at 20℃; | 95% |
| With bromide; hydrogen cation; cis-nitrous acid In water at 25℃; Rate constant; also in the presence of SCN- ions; var. conc. of the malonamide, Br and SCN ions; | |
| With water; acetic acid; sodium nitrite | |
| With nitrosylchloride |
-
-
108-13-4
malonodiamide

-
-
31217-00-2
5-chloro-1,3-dimethyluracil

-
-
75993-42-9
5-Chloro-2-hydroxy-6-oxo-1,6-dihydro-pyridine-3-carboxylic acid amide

| Conditions | Yield |
|---|---|
| 95% |

| Conditions | Yield |
|---|---|
| In methanol; ethanol refluxing (8 h); crystals deposited by cooling the soln., washed several times with diethyl ether; elem. anal.; | 95% |
-
-
108-13-4
malonodiamide

-
-
1072027-60-1
1-butoxy-4,4,5,5,5-pentafluoropent-1-en-3-one

-
-
1072021-29-4
2-Oxo-6-(pentafluoroethyl)-1,2-dihydropyridine-3-carboxamide

| Conditions | Yield |
|---|---|
| With ethanol; sodium for 7h; Heating / reflux; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 150℃; for 1h; Microwave irradiation; | 92% |
| With 1,2,3-trichlorobenzene | |
| With ethylene glycol |

| Conditions | Yield |
|---|---|
| With ethanol; sodium for 3h; Reflux; | 91% |
| With sodium ethanolate In ethanol for 2h; Inert atmosphere; Reflux; | 26% |
-
-
108-13-4
malonodiamide

-
-
63650-28-2
9-benzylidene-9,10-dihydroanthracene

-
A
-
96784-21-3
9-(α-Acetoxybenzylidene)-9,10-dihydroanthracene

-
B
-
96784-20-2
9-Acetoxy-9-benzoyl-9,10-dihydroanthracene

-
C
-
96784-19-9
4'-Carbamoyl-3'-phenyl-1',5'-dihydrospiropyrrol>-5'-one

| Conditions | Yield |
|---|---|
| With manganese triacetate; acetic acid for 0.00333333h; Heating; | A 5% B 2% C 90% |

-
-
108-13-4
malonodiamide

-
-
27895-67-6
N-(3,4-dimethoxybenzylidene)aniline

-
-
86213-21-0
3,4-dimethoxybenzalmalonamide

| Conditions | Yield |
|---|---|
| With piperidine In benzene Ambient temperature; | 90% |
-
-
108-13-4
malonodiamide

-
-
109317-78-4
4-n-butoxy-1,1,1-trifluorobut-3-en-2-one

-
-
116548-03-9
2-oxo-6-(trifluoromethyl)-1,2-dihydropyridine-3-carboxamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium ethanolate In ethanol Heating; | 90% |
-
-
108-13-4
malonodiamide

-
-
10128-99-1
3-benzyl-6-methyl-[1,3]oxazine-2,4-dione

-
-
141946-12-5
3-benzyl-5-methylpyrido<4,3-d>pyrimidine-2,4,7(1H,3H,6H)-trione

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate In N,N-dimethyl-formamide at 40 - 50℃; for 3.5h; | 90% |

| Conditions | Yield |
|---|---|
| With bromine In acetic acid at 60℃; for 5h; | 89% |
| With perchloric acid; iodine In water at 25℃; Rate constant; var. HClO4 conc.; | |
| With bromine; acetic acid | |
| With dibromomalononitrile; boron trifluoride In ethanol Heating; | |
| With bromine In acetic acid |
-
-
108-13-4
malonodiamide

-
-
78999-59-4
1-cyclohexyl-3-methyl-5-nitrouracil

-
-
37721-35-0
5-carbamoyl-1-cyclohexyluracil

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 1h; Heating; | 89% |
| With sodium ethanolate In ethanol for 1h; Heating; | 86% |
-
-
108-13-4
malonodiamide

-
-
78999-60-7
3-cyclohexyl-1-methyl-5-nitrouracil

-
-
78999-61-8
5-carbamoyl-1-methyluracil

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 2h; Heating; | 89% |
| With sodium ethanolate In ethanol for 1h; Heating; | 86% |
-
-
108-13-4
malonodiamide

-
-
1846-76-0
ethyl coumarin-3-carboxylate

-
-
1846-78-2
2-oxo-2H-chromene-3-carboxamide

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 6h; Michael reaction; Heating; | 88% |
-
-
108-13-4
malonodiamide

-
-
1244024-26-7
1-phenyl-3-(1-methyl-4,5,6,7-tetrahydro-1H-indol-2-yl)-2-propynone

| Conditions | Yield |
|---|---|
| Stage #1: malonodiamide With potassium hydroxide In dimethyl sulfoxide at 20 - 25℃; for 0.5h; Stage #2: 1-phenyl-3-(1-methyl-4,5,6,7-tetrahydro-1H-indol-2-yl)-2-propynone In dimethyl sulfoxide at 20 - 25℃; for 24h; | 88% |
-
-
108-13-4
malonodiamide

-
-
20413-06-3, 88137-32-0, 88137-31-9
(E)-2-benzoyl-3-(4-methoxyphenyl)acrylonitrile

-
-
100784-42-7
3-carbamoyl-5-cyano-6-hydroxy-4-(p-methoxyphenyl)-6-phenylpiperidin-2-one

| Conditions | Yield |
|---|---|
| With piperidine In methanol for 16h; Ambient temperature; | 87% |
-
-
108-13-4
malonodiamide

-
-
69645-51-8
2-nitromalonamide

| Conditions | Yield |
|---|---|
| With nitric acid; acetic acid at 15 - 20℃; for 1h; | 86% |
| With nitric acid | |
| With sulfuric acid; nitric acid |

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide under 15.0015 - 37.5038 Torr; for 1h; Temperature; Heating; | 86% |
| With phosphorus pentaoxide | |
| With phosphorus pentoxide at 250℃; under 15001.5 - 37503.8 Torr; for 1h; Temperature; | |
| under 15.0015 - 37.5038 Torr; for 1h; Temperature; Heating; |
-
-
108-13-4
malonodiamide

-
-
20413-05-2, 101220-34-2, 101220-25-1
(E)-2-Benzoyl-3-phenyl-acrylonitrile

-
-
75238-88-9
3-carbamoyl-5-cyano-6-hydroxy-4,6-diphenylpiperidin-2-one

| Conditions | Yield |
|---|---|
| With piperidine In methanol for 16h; Ambient temperature; | 86% |
| With piperidine In methanol |
-
-
108-13-4
malonodiamide

-
-
919-30-2
3-aminopropyltriethoxysilane

-
-
223476-24-2
N,N'-bis(3-triethoxysilylpropyl)malonic amide

| Conditions | Yield |
|---|---|
| With ammonium sulfate at 145 - 150℃; for 21h; | 86% |

| Conditions | Yield |
|---|---|
| With piperidine In ethanol Michael reaction; Heating; | 86% |
-
-
108-13-4
malonodiamide

-
-
73003-80-2
2,2-dibromomalonamide

| Conditions | Yield |
|---|---|
| With lead(IV) acetate; zinc dibromide In acetonitrile at 20℃; for 0.25h; | 85% |
| With water; bromine at 70 - 80℃; | |
| With bromine |
-
-
41613-26-7
1,3-dimethyl-5-nitrouracil

-
-
108-13-4
malonodiamide

-
A
-
72078-82-1
N-methylnitroacetamide

-
B
-
78999-61-8
5-carbamoyl-1-methyluracil

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 1h; Heating; other derivatives; | A n/a B 84% |
| With sodium ethanolate In ethanol for 1h; Product distribution; Mechanism; Heating; | A 0.02 g B 84% |
| With sodium ethanolate In ethanol for 1h; Heating; | A n/a B 84% |
| With sodium ethanolate In ethanol for 1h; Heating; | A 0.02 g B 84% |

Malonamide Chemical Properties
Molecular Structure of Malonamide (CAS NO. 108-13-4):
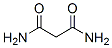
IUPAC Name: Propanediamide
Product Categories: API intermediates
Molecular Formula: C3H6N2O2
Molecular Weight: 102.091940 g/mol
XLogP3-AA: -1.8
H-Bond Donor: 2
H-Bond Acceptor: 2
Canonical SMILES: C(C(=O)N)C(=O)N
InChI: InChI=1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7)
InChIKey: WRIRWRKPLXCTFD-UHFFFAOYSA-N
Surface Tension: 57.8 dyne/cm
Density: 1.281 g/ml
Flash Point: 232.5 °C
Enthalpy of Vaporization: 72.16 kJ/mol
Boiling Point: 460.9 °C at 760 mmHg
Vapour Pressure: 1.13E-08 mmHg at 25°C
Water Solubility: 180 g/L (20 oC)
Melting Point: 172-175 °C(lit.)
BRN: 1751401
EINECS: 203-553-8
Malonamide Toxicity Data With Reference
| 1. | orl-mus LD50:6000 mg/kg | BIJOAK Biochemical Journal. 34 (1940),1196. |
Malonamide Consensus Reports
Reported in EPA TSCA Inventory.
Malonamide Safety Profile
Safety information of Malonamide(108-13-4):
Safety Statements: 22-24/25
S22: Do not breathe dust
S24/25:Avoid contact with skin and eyes
WGK Germany: 2
RTECS: ON9125000
HS Code: 29241900
Mildly toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx.
Malonamide Specification
Malonamide with cas registry number of 108-13-4 is stable under normal temperatures and pressures but incompatible with strong oxidizing agents, strong reducing agents, acids, bases. It is also known as AI3-24284 ; Carboxamidoacetamide ; Malondiamide ; Malonic acid diamide ; Malonic diamide ; Malonodiamide ; Malonyldiamide ; NSC 2134 ; Propanediamide . Malonamide is mainly used as raw material in organic synthesis.
Related Products
- Malonamide
- 1081-34-1
- 10813-74-8
- 108138-46-1
- 108141-35-1
- 108149-60-6
- 108149-63-9
- 108149-65-1
- 108153-74-8
- 108-15-6
- 108166-02-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View