-
Name
Megestrol acetate
- EINECS 209-864-5
- CAS No. 595-33-5
- Article Data30
- CAS DataBase
- Density 1.15 g/cm3
- Solubility
- Melting Point 214 °C
- Formula C24H32O4
- Boiling Point 507.1 °C at 760 mmHg
- Molecular Weight 384.516
- Flash Point 218.5 °C
- Transport Information
- Appearance Crystalline Solid
- Safety 22-24/25
- Risk Codes 40-48
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Megace ES;Pregna-4,6-diene-3,20-dione, 17-(acetyloxy)-6-methyl-;Ovarid;Megace;Ovaban (Veterinary);Megeron;6-Methyl-delta4,6-pregnadien-17alpha-ol-3,20-dione acetate;6-Methyl-6-dehydro-17.alpha.-acetoxyprogesterone;Megace (TN);SC 10363;Megestryl acetate;Pregna-4,6-diene-3, 20-dione, 17-hydroxy-6-methyl-, acetate;Pallace;Ovaban;S4;6-Methyl-17-alpha-hydroxy-delta(sup 6)-progesterone acetate;SC-10363;SC10363;Niagestin;Progesterone, 6-dehydro-17-hydroxy-6-methyl-, acetate;6-Methyl-delta(sup 4,6)-pregnadien-17-alpha-ol-3,20-dione acetate;Megestrol acetate [USAN];17alpha-Acetoxy-6-dehydro-6-methylprogesterone;Megestrol acetate (USAN);Megestat;6-Dehydro-6-methyl-17.alpha.-acetoxyprogesterone;17-Hydroxy-6-methylpregna-4,6-diene-3,20-dione acetate;Megestrol acetole [Progestins];6-MethleneProgesterone Acetate;Pregna-4,6-diene-3, 20-dione, 17- (acetyloxy)-6-methyl-;BDH 1298;
- PSA 60.44000
- LogP 4.57530
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: methyl bromide; C27H38O7 With magnesium In tetrahydrofuran at 30 - 55℃; Inert atmosphere; Stage #2: With hydrogenchloride In water at 60 - 65℃; | 69.8% |
-
-
4382-76-7
methoxymethyl acetate

-
-
4134-58-1, 17308-02-0, 41460-93-9, 41460-97-3, 630-56-8, 302-23-8
hydroxyprogesterone acetate

-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| With sodium acetate; trichlorophosphate In chloroform at 70℃; for 0.166667h; | 51% |
| With sodium acetate; trichlorophosphate In chloroform |

| Conditions | Yield |
|---|---|
| With chloranil | |
| With 2-methyl-propan-1-ol; chloranil Heating; | |
| With chloranil | |
| Multi-step reaction with 2 steps 1: TsOH / dioxane 2: (tBuO)2CrO2, Ac2O / CCl4 View Scheme |
-
-
71-58-9
Medroxyprogesterone acetate

-
-
118-75-2
chloranil

-
-
64-19-7
acetic acid

-
-
141-78-6
ethyl acetate

-
-
595-33-5
Megestrol acetate

-
-
71-58-9
Medroxyprogesterone acetate

-
-
118-75-2
chloranil

-
-
75-65-0
tert-butyl alcohol

-
-
595-33-5
Megestrol acetate


| Conditions | Yield |
|---|---|
| With acetic anhydride In tetrachloromethane |

| Conditions | Yield |
|---|---|
| With aluminum tri-tert-butoxide; p-benzoquinone |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: benzene; toluene-4-sulfonic acid 2: peroxybenzoic acid; chloroform 3: benzene; diethyl ether 4: aqueous methanol.H2SO4 5: CrO3; pyridine 6: aqueous HCl 7: acetic acid; toluene-4-sulfonic acid 8: tetrachloro-<1,4>benzoquinone View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid; toluene-4-sulfonic acid 2: tetrachloro-<1,4>benzoquinone View Scheme |
-
-
113665-92-2
3β,5,17-trihydroxy-6β-methyl-5α-pregnan-20-one

-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: CrO3; pyridine 2: aqueous HCl 3: acetic acid; toluene-4-sulfonic acid 4: tetrachloro-<1,4>benzoquinone View Scheme |
-
-
23706-51-6
5,17-dihydroxy-6β-methyl-5α-pregnane-3,20-dione

-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous HCl 2: acetic acid; toluene-4-sulfonic acid 3: tetrachloro-<1,4>benzoquinone View Scheme |
-
-
19699-76-4
20,20-ethanediyldioxy-6β-methyl-5α-pregnane-3β,5,17-triol

-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: aqueous methanol.H2SO4 2: CrO3; pyridine 3: aqueous HCl 4: acetic acid; toluene-4-sulfonic acid 5: tetrachloro-<1,4>benzoquinone View Scheme |
-
-
19699-74-2
3β-acetoxy-20,20-ethanediyldioxy-pregn-5-en-17-ol

-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: peroxybenzoic acid; chloroform 2: benzene; diethyl ether 3: aqueous methanol.H2SO4 4: CrO3; pyridine 5: aqueous HCl 6: acetic acid; toluene-4-sulfonic acid 7: tetrachloro-<1,4>benzoquinone View Scheme |
-
-
19699-75-3
3β-acetoxy-20,20-ethanediyldioxy-5,6α-epoxy-5α-pregnan-17-ol

-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: benzene; diethyl ether 2: aqueous methanol.H2SO4 3: CrO3; pyridine 4: aqueous HCl 5: acetic acid; toluene-4-sulfonic acid 6: tetrachloro-<1,4>benzoquinone View Scheme |
-
-
32634-95-0
17α-Acetoxy-6-methylene-pregn-4-ene-3,20-dione

-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| With sodium acetate; palladium on activated charcoal; 4-methylcyclohexene In ethanol Heating / reflux; |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran; water at 20℃; for 5h; | 97% |
| With sodium tetrahydroborate In methanol for 0.5h; | 94.5% |
| With lithium tri(t-butoxy)aluminum hydride In tetrahydrofuran at 0℃; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water for 1.5h; Heating; | 94% |
| With sodium methylate | |
| With sodium hydroxide In methanol; water |
-
-
595-33-5
Megestrol acetate

| Conditions | Yield |
|---|---|
| With bunazosin In 1,4-dioxane; ethyl acetate | 90% |
Megestrol acetate Chemical Properties
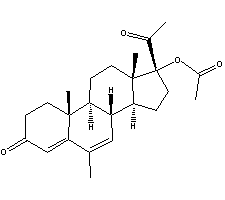
Name:17-Acetyloxy-6-methylpregna-4,6-diene-3,20-dione
Other names:Megestrol acetate;6-diene-3,20-dione;17-hydroxy-6-methyl-pregna-acetate;17- (acetyloxy)-6-methylpregna-4 ,6-dien-3,20-dione;17-acetoxy-6-methylpregna-4, 6-dien-3;20-dione;megestrol acetate acetate; 17α-acetoxy-6-methyl-pregn-4 ,6-diene-;6-methyl-pregn-4 ,6-dien-17 alpha-ol ,20 - dione-17-acetate
17α-hydroxy-6-methyl-;16-4,6-diene; 20-dione-17-acetate
Molecular formula : C24H32O4
MW : 384.52
refractive index: 11°C (C=2, CHCl3)
Melting point:214-216 °C
ACD/LogP(pH 5.5): 3.82
ACD/LogP(pH 5.5): 3.82
ACDXLogP: 3.90
nature : a white or slightly yellow crystalline powder in methanol, tasteless, odorless.Soluble acetone, ethanol-soluble, ether, do not dissolve in water.
Megestrol acetate Uses
Megestrol acetate Production
available 16-17α-dione by the open-loop hydrogen bromide and nickel Catalytic Reduction of bromine, in the production of 17 alpha-hydroxy progesterone, and then acetylated and acid hydrolysis of the production of 17 alpha-acetoxy progesterone, and then to the original three formic acid and ethyl ether of Vilsmeier reacted 3-ethoxy-6-formyl-17α- acetoxy ,16 ,5-diene-20-one, and the final KBH4, acid hydrolysis, dehydration and transposition of the goods obtained.
Megestrol acetate Toxicity Data With Reference
| 1. | dns-mus-scu 200 mg/kg | JOENAK Journal of Endocrinology. 60 (1974),167. | ||
| 2. | dni-mus-scu 200 mg/kg | JOENAK Journal of Endocrinology. 60 (1974),167. | ||
| 3. | ivn-mus LD50:56 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#00931 . |
Megestrol acetate Consensus Reports
Megestrol acetate Safety Profile
Megestrol acetate Standards and Recommendations
Megestrol Acetate, 99%
Megestrol acetate, 98%
Related Products
- Megestrol
- Megestrol acetate
- Megestrol acetate mixed with ethinyloestradiol
- 59536-17-3
- 59536-65-1
- 5953-76-4
- 595-37-9
- 595-40-4
- 59541-83-2
- 595-46-0
- 5954-65-4
- 59548-39-9
- 59549-51-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View