-
Name
Menatetrenone
- EINECS
- CAS No. 863-61-6
- Article Data11
- CAS DataBase
- Density 0.994 g/cm3
- Solubility
- Melting Point 350 °C
- Formula C31H40O2
- Boiling Point 570.6 °C at 760 mmHg
- Molecular Weight 444.657
- Flash Point 208.3 °C
- Transport Information
- Appearance yellow crystals
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Vitamin K2(mk-4);1,4-Naphthalenedione,2-methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-, (E,E,E)-;1,4-Naphthalenedione,2-methyl-3-[(2E,6E,10E)-3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl]-(9CI);2-Methyl-3-geranylgeranyl-1,4-naphthoquinone;Glakay;Kaytwo;MK4;Menaquinone4;Menaquinone K4;Vitamin K2(20);Vitamin MK 4;
- PSA 34.14000
- LogP 8.91790
Synthetic route
-
-
66958-54-1
(2'E,6'E,10'E,14'E)-2-(3',7',11',15'-tetramethylhexadeca-2',6',10',14'-tetraenyl)-1,4-dimethoxy-3-methylnaphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate In water; acetonitrile at 10 - 20℃; for 1h; | 90.6% |
| With ammonium cerium(IV) nitrate; triisooctyl amine In hexane; water; acetonitrile for 0.5h; | 86% |
| With ammonium cerium(IV) nitrate In dichloromethane; acetonitrile at 0℃; for 0.5h; | 72% |
-
-
94827-99-3
2-<9'-(2-pyridylthio)-tetraprenyl>-3-methyl-1,4-dimethoxynaphthalene

-
A
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; ammonium cerium(IV) nitrate; lithium methanolate; copper dichloride 1.) THF, 1 h, reflux; 2.) CH2Cl2, CH3CN, H2O, 0 deg C; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
94828-03-2
2-(5'-p-tosyl-tetraprenyl)-3-methyl-1,4-dibenzyloxynaphthalene

-
A
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With oxygen; lithium; ethylamine 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O; Yield given. Multistep reaction; |
-
-
94828-02-1
2-(9'-p-tosyl-tetraprenyl)-3-methyl-1,4-dibenzyloxynaphthalene

-
A
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With oxygen; lithium; ethylamine 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O; Yield given. Multistep reaction; |
-
-
197071-04-8
4-N,N-dimethylglycyloxy-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With isotonic phosphate buffer; rat liver homogenate at 37℃; pH=7.4; Kinetics; Further Variations:; Reagents; Hydrolysis; |
-
-
197071-03-7
1-N,N-dimethylglycyloxy-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With isotonic phosphate buffer; rat liver homogenate at 37℃; pH=7.4; Kinetics; Further Variations:; Reagents; Hydrolysis; |
-
-
197071-05-9
1,4-bis(N,N-dimethylglycyloxy)-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

-
A
-
863-61-6
menatetrenone

-
B
-
197071-04-8
4-N,N-dimethylglycyloxy-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

-
C
-
197071-03-7
1-N,N-dimethylglycyloxy-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

| Conditions | Yield |
|---|---|
| With isotonic phosphate buffer; rat liver homogenate at 37℃; pH=7.4; Kinetics; Further Variations:; Reagents; Hydrolysis; |


-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With male Wistar rat liver microsome preparation In water at 25℃; Enzyme kinetics; Hydrolysis; Enzymatic reaction; |

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With male Wistar rat liver microsome preparation In water at 25℃; Enzyme kinetics; Hydrolysis; Enzymatic reaction; |

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With male Wistar rat liver microsome preparation In water at 25℃; Enzyme kinetics; Hydrolysis; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; zinc(II) chloride anschliessendes Behandeln mit Ag2O; |
-
-
24163-93-7, 24163-94-8, 56881-57-3, 6874-67-5, 28290-41-7
farnesyl bromide

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / t-BuOK / tetrahydrofuran / 5 h / -20 °C 2: 95 percent / Pd(dppe)Cl2; LiEt3BH / tetrahydrofuran / 1 h / 0 °C 3: 72 percent / aq. CAN / CH2Cl2; acetonitrile / 0.5 h / 0 °C View Scheme |
-
-
73875-18-0
(2′E)-2-(3′-methyl-4′-phenylsulfonylbut-2′-enyl)-1,4-dimethoxy-3-methylnaphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / t-BuOK / tetrahydrofuran / 5 h / -20 °C 2: 95 percent / Pd(dppe)Cl2; LiEt3BH / tetrahydrofuran / 1 h / 0 °C 3: 72 percent / aq. CAN / CH2Cl2; acetonitrile / 0.5 h / 0 °C View Scheme |
-
-
618457-08-2
(2'E,6'E,10'E,14'E)-2-(4'-phenylsulfonyl-3',7',11',15'-tetramethylhexadeca-2',6',10',14'-tetraenyl)-1,4-dimethoxy-3-methylnaphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / Pd(dppe)Cl2; LiEt3BH / tetrahydrofuran / 1 h / 0 °C 2: 72 percent / aq. CAN / CH2Cl2; acetonitrile / 0.5 h / 0 °C View Scheme |
-
-
53772-33-1
2-bromo-3-methyl-1,4-dimethoxynaphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 58 percent / n-BuLi / diethyl ether / 1.) 0 deg , 10 min; 1 h, 2.) 0 deg C, 30 min 2: 2.) THF, 0-5 deg C, 10 min, sonication 3: 86 percent / cerium ammonium nitrate, Adogen / acetonitrile; H2O; hexane / 0.5 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: n-butyllithium / tetrahydrofuran; hexane / 1 h / -78 °C / Inert atmosphere 1.2: 0.5 h / -78 °C / Inert atmosphere 2.1: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / N,N-dimethyl-formamide / 15 h / 70 °C 3.1: ammonium cerium (IV) nitrate / acetonitrile; water / 1 h / 10 - 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / 1,4-dioxane / 10 h / 100 °C 2: potassium acetate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 15 h / 100 °C 3: ammonium cerium (IV) nitrate / acetonitrile; water / 1 h / 10 - 20 °C View Scheme |
-
-
132513-59-8
(2Ξ,6E,10E)-3,7,11,15-tetramethyl-hexadeca-2,6,10,14-tetraenal

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 58 percent / n-BuLi / diethyl ether / 1.) 0 deg , 10 min; 1 h, 2.) 0 deg C, 30 min 2: 2.) THF, 0-5 deg C, 10 min, sonication 3: 86 percent / cerium ammonium nitrate, Adogen / acetonitrile; H2O; hexane / 0.5 h View Scheme |
-
-
156840-09-4
(2'E,6'E,10'E)-2-Methyl-3-(1'-hydroxy-3',7',11',15'-tetramethyl-2',6',10',14'-hexadecatetraenyl)-1,4-dimethoxynaphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2.) THF, 0-5 deg C, 10 min, sonication 2: 86 percent / cerium ammonium nitrate, Adogen / acetonitrile; H2O; hexane / 0.5 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 85 percent / dimethylformamide / 16 h / Ambient temperature 2: 75 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 3: 1.) CuCl2, LiAlH4, MeOLi; 2.) ceric ammonium nitrate / 1.) THF, 1 h, reflux; 2.) CH2Cl2, CH3CN, H2O, 0 deg C View Scheme |
-
-
53254-60-7
3,7-dimethyl-1-(p-toluenesulfonyl)-2(E),6-octadiene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 2: 1.) Li in EtNH2; 2.) oxygen / 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O View Scheme |
-
-
68690-45-9, 80370-68-9, 53254-63-0
(2E,6E)-3,7,11-trimethyl-1-(p-tolylsulfonyl)dodeca-2,6,10-triene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 87 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 2: 1.) Li in EtNH2; 2.) oxygen / 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O View Scheme |
-
-
82895-40-7
2-[(2E)-3,7-dimethyl-2,6-octadienyl]sulfanylpyridine

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 2: 1.) CuCl2, LiAlH4, MeOLi; 2.) ceric ammonium nitrate / 1.) THF, 1 h, reflux; 2.) CH2Cl2, CH3CN, H2O, 0 deg C View Scheme |
-
-
94828-13-4
2-(8-bromo-3,7-dimethyl-octa-2,6-dienyl)-1,4-dimethoxy-3-methyl-naphtalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 2: 1.) CuCl2, LiAlH4, MeOLi; 2.) ceric ammonium nitrate / 1.) THF, 1 h, reflux; 2.) CH2Cl2, CH3CN, H2O, 0 deg C View Scheme |
-
-
94828-05-4
8-(1,4-dimethoxy-3-methyl-naphtalen-2-yl)-2,6-dimethyl-octa-2,6-dien-1-ol

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: PBr3 / diethyl ether / 1.5 h / 0 °C 2: 75 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 3: 1.) CuCl2, LiAlH4, MeOLi; 2.) ceric ammonium nitrate / 1.) THF, 1 h, reflux; 2.) CH2Cl2, CH3CN, H2O, 0 deg C View Scheme |
-
-
94828-30-5
(E)-4-(1,4-Bis-benzyloxy-3-methyl-naphthalen-2-yl)-2-methyl-but-2-en-1-ol

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: PBr3 / diethyl ether / 1.5 h / 0 °C 2: 87 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 3: 1.) Li in EtNH2; 2.) oxygen / 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O View Scheme |
-
-
94828-04-3
1,4-Bis-benzyloxy-2-((E)-4-bromo-3-methyl-but-2-enyl)-3-methyl-naphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 87 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 2: 1.) Li in EtNH2; 2.) oxygen / 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O View Scheme |
-
-
94828-06-5
(2E,6E)-8-(1,4-Bis-benzyloxy-3-methyl-naphthalen-2-yl)-2,6-dimethyl-octa-2,6-dien-1-ol

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: PBr3 / diethyl ether / 1.5 h / 0 °C 2: 86 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 3: 1.) Li in EtNH2; 2.) oxygen / 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O View Scheme |
-
-
94828-14-5
1,4-Bis-benzyloxy-2-((2E,6E)-8-bromo-3,7-dimethyl-octa-2,6-dienyl)-3-methyl-naphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86 percent / 1.) hexamethylphosphoric triamide, BuLi / tetrahydrofuran; hexane / -70 - 0 °C 2: 1.) Li in EtNH2; 2.) oxygen / 1.) THF, -70 deg C; 2.) THF, MeOH, H2O, Et2O View Scheme |
-
-
39776-45-9
menahydroquinone-4

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| With oxygen; sodium chloride In hexane; water; ethyl acetate; toluene at 25 - 40℃; for 3h; Product distribution / selectivity; | |
| With oxygen In water; toluene at 30 - 60℃; for 15h; Product distribution / selectivity; |
-
-
53772-19-3
1,4-dimethoxy-2-methylnaphthalene

-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: bromine / dichloromethane / 2 h / 10 °C 2.1: n-butyllithium / tetrahydrofuran; hexane / 1 h / -78 °C / Inert atmosphere 2.2: 0.5 h / -78 °C / Inert atmosphere 3.1: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / N,N-dimethyl-formamide / 15 h / 70 °C 4.1: ammonium cerium (IV) nitrate / acetonitrile; water / 1 h / 10 - 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: bromine / dichloromethane / 2 h / 10 °C 2: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / 1,4-dioxane / 10 h / 100 °C 3: potassium acetate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 15 h / 100 °C 4: ammonium cerium (IV) nitrate / acetonitrile; water / 1 h / 10 - 20 °C View Scheme |
-
-
863-61-6
menatetrenone

-
-
39776-45-9
menahydroquinone-4

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol; di-isopropyl ether for 0.166667h; Reduction; |
-
-
863-61-6
menatetrenone

| Conditions | Yield |
|---|---|
| In acetonitrile at 20 - 22℃; Flash photolysis; |
-
-
863-61-6
menatetrenone

-
-
197071-04-8
4-N,N-dimethylglycyloxy-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaBH4 / diisopropyl ether; methanol / 0.17 h 2: dicyclohexylcarbodiimide; pyridine / 24 h / 20 °C View Scheme |
-
-
863-61-6
menatetrenone

-
-
197071-03-7
1-N,N-dimethylglycyloxy-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaBH4 / diisopropyl ether; methanol / 0.17 h 2: dicyclohexylcarbodiimide; pyridine / 24 h / 20 °C View Scheme |
-
-
863-61-6
menatetrenone

-
-
197071-05-9
1,4-bis(N,N-dimethylglycyloxy)-2-methyl-3-tetraprenyl-4-hydroxy-naphthalene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaBH4 / diisopropyl ether; methanol / 0.17 h 2: dicyclohexylcarbodiimide; pyridine / 24 h / 20 °C View Scheme |
Menatetrenone Chemical Properties
The molecular structure of Menatetrenone (CAS NO.863-61-6):
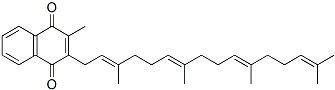
IUPAC Name: 2-Methyl-3-[(2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraenyl]naphthalene-1,4-dione
Molecular Weight: 444.6481 g/mol
Molecular Formula: C31H40O2
Density: 0.994 g/cm3
Melting Point: 350 °C
Boiling Point: 570.6 °C at 760 mmHg
Flash Point: 208.3 °C
Index of Refraction: 1.538
Molar Refractivity: 139.92 cm3
Molar Volume: 446.8 cm3
Surface Tension: 36.7 dyne/cm
Enthalpy of Vaporization: 85.57 kJ/mol
Vapour Pressure: 4.97E-13 mmHg at 25 °C
Storage Temp.: −20 °C
XLogP3: 8.9
H-Bond Acceptor: 2
Rotatable Bond Count: 11
Tautomer Count: 27
Exact Mass: 444.302831
MonoIsotopic Mass: 444.302831
Topological Polar Surface Area: 34.1
Heavy Atom Count: 33
Canonical SMILES: CC1=C(C(=O)C2=CC=CC=C2C1=O)CC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)C
Isomeric SMILES: CC1=C(C(=O)C2=CC=CC=C2C1=O)C/C=C(\C)/CC/C=C(\C)/CC/C=C(\C)/CCC=C(C)C
InChI: InChI=1S/C31H40O2/c1-22(2)12-9-13-23(3)14-10-15-24(4)16-11-17-25(5)20-21-27-26(6)30(32)28-18-7-8-19-29(28)31(27)33/h7-8,12,14,16,18-20H,9-11,13,15,17,21H2,1-6H3/b23-14+,24-16+,25-20+
InChIKey: DKHGMERMDICWDU-GHDNBGIDSA-N
Product Categories: Vitamins and derivatives; Osteoporosis; Intermediates & Fine Chemicals; Pharmaceuticals
Menatetrenone Uses
Menatetrenone (CAS NO.863-61-6) is used as Vitamin (prothrombogenic). It is also used as a hemostatic agent and as adjunctive therapy for the pain of osteoporosis.
Menatetrenone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD | oral | > 1gm/kg (1000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 445, 1971. | |
| dog | LD | unreported | > 3gm/kg (3000mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 37, Pg. 2518, 1995. | |
| dog | LD50 | intravenous | > 40mL/kg (40mL/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BLOOD: PIGMENTED OR NUCLEATED RED BLLOD CELLS | Kiso to Rinsho. Clinical Report. Vol. 25, Pg. 2003, 1991. |
| monkey | LD50 | intravenous | > 10mL/kg (10mL/kg) | BLOOD: CHANGES IN CELL COUNT (UNSPECIFIED) | Kiso to Rinsho. Clinical Report. Vol. 25, Pg. 2003, 1991. |
| mouse | LD | oral | > 5mg/kg (5mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 445, 1971. | |
| mouse | LD50 | intravenous | > 40mL/kg (40mL/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Kiso to Rinsho. Clinical Report. Vol. 25, Pg. 2003, 1991. |
| mouse | LDLo | intraperitoneal | 5gm/kg (5000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 445, 1971. | |
| mouse | LDLo | subcutaneous | 5gm/kg (5000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 445, 1971. | |
| rabbit | LD | intravenous | > 100mg/kg (100mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 461, 1971. | |
| rabbit | LDLo | subcutaneous | 100mg/kg (100mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 461, 1971. |
| rat | LD | oral | > 5gm/kg (5000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 445, 1971. | |
| rat | LD | subcutaneous | > 5gm/kg (5000mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 445, 1971. |
| rat | LD50 | intravenous | > 40mL/kg (40mL/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Kiso to Rinsho. Clinical Report. Vol. 25, Pg. 2003, 1991. |
| rat | LDLo | intraperitoneal | 5gm/kg (5000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 5, Pg. 445, 1971. |
Menatetrenone Safety Profile
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
RTECS: QL9279500
An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
Menatetrenone Specification
Menatetrenone (CAS NO.863-61-6) is also named as 2-Methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-1,4-naphthochinon ; 2-Methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-1,4-naphthoquinone ; 2-Methyl-3-geranylgeranyl-1,4-naphthoquinone ; 2-Methyl-3-trans-tetraprenyl-1,4-naphthoquinone ; E3100 ; K2(sub 20) ; Kaytwo ; Kephton ; MK4 ; Menaquinone K4 ; Menaquinone-4 ; Menatetrenona ; Menatetrenona [INN-Spanish] ; Menatetrenonum ; Menatetrenonum [INN-Latin] ; UNII-27Y876D139 ; Vitamin K2(sub 20) ; Vitamin MK 4 . Menatetrenone (CAS NO.863-61-6) is yellow crystals.
Related Products
- Menatetrenone
- 863643-48-5
- 86366-52-1
- 863668-07-9
- 863678-76-6
- 863752-59-4
- 86375-28-2
- 863753-30-4
- 863753-35-9
- 863-76-3
- 86376-49-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View