-
Name
Methyl Bromopyruvate
- EINECS
- CAS No. 7425-63-0
- Article Data10
- CAS DataBase
- Density 1.70
- Solubility
- Melting Point 5°C(lit.)
- Formula C4H5 Br O3
- Boiling Point 186.8oC at 760 mmHg
- Molecular Weight 180.986
- Flash Point 125oC
- Transport Information
- Appearance
- Safety S26;S36
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols

- Synonyms Pyruvicacid, bromo-, methyl ester (6CI,7CI,8CI); Methyl 3-bromo-2-oxopropanoate;Methyl bromopyruvate; NSC 526776
- PSA 43.37000
- LogP 0.12340
Synthetic route
-
-
27871-49-4
(S)-Methyl lactate

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane for 6h; Reflux; | 61% |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; bromine am Licht; | |
| With pyridine; bromine at 40 - 50℃; | |
| With bromine at 65℃; unter Durchleiten von CO2; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; for 15h; | |
| hydrogenchloride In water at 30 - 40℃; for 9h; |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

| Conditions | Yield |
|---|---|
| With pyridine In ethanol at 80℃; for 3.5h; Inert atmosphere; | 100% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
101924-70-3
(R)-methyl 2-(1-(tert-butoxycarbonylamino)-2-phenylethyl)thiazole-4-carboxylate

| Conditions | Yield |
|---|---|
| With calcium carbonate In ethanol at 20℃; Hantzsch cyclization; Inert atmosphere; | 99% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
17356-08-0
thiourea

-
-
118452-04-3
methyl 2-amino-1,3-thiazole-4-carboxylate

| Conditions | Yield |
|---|---|
| In methanol for 2h; Hantzsch Thiazole Synthesis; Reflux; | 93% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
73956-15-7
2,2-diethoxythioacetamide

| Conditions | Yield |
|---|---|
| In methanol for 1.5h; Molecular sieve; Reflux; | 91% |
| With hydrogenchloride In methanol; water at 65℃; for 3.25h; Molecular sieve; |

| Conditions | Yield |
|---|---|
| In toluene Reflux; | 90% |

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
71368-35-9
6-methoxybenzo[d]thiazole-2-carbothioamide

-
-
7775-89-5
2-(6-methoxy-benzothiazol-2-yl)-thiazole-4-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 39h; Reflux; | 88% |
| In methanol |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-bromo-2-oxopropanoate; (S)-N-(tert-butoxycarbonyl)-O-(tert-butyldiphenylsilyl)serinethioamide With potassium hydrogencarbonate In 1,2-dimethoxyethane at 0 - 20℃; Stage #2: With 2,6-dimethylpyridine; trifluoroacetic anhydride In 1,2-dimethoxyethane at 0 - 20℃; | 88% |
| Stage #1: methyl 3-bromo-2-oxopropanoate; (S)-N-(tert-butoxycarbonyl)-O-(tert-butyldiphenylsilyl)serinethioamide With potassium hydrogencarbonate In 1,2-dimethoxyethane at 0 - 20℃; for 3h; Stage #2: With 2,6-dimethylpyridine; trifluoroacetic anhydride In 1,2-dimethoxyethane at 0℃; for 1h; | 88% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
246265-78-1
(R)-3-t-butoxycarbonyl-2,2-dimethylthiazolidine-4-carbothioamide

-
-
924288-22-2
2',2'-dimethyl-4',5'-dihydro-[2,4']bithiazolyl-4,3'-dicarboxylic acid 3'-tert-butyl ester 4-methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-bromo-2-oxopropanoate; (R)-3-t-butoxycarbonyl-2,2-dimethylthiazolidine-4-carbothioamide With potassium hydrogencarbonate In tetrahydrofuran at 0 - 20℃; for 22h; Inert atmosphere; Stage #2: With pyridine; trifluoroacetic anhydride In tetrahydrofuran at 0℃; for 2.5h; Inert atmosphere; | 86% |
| Stage #1: methyl 3-bromo-2-oxopropanoate; (R)-3-t-butoxycarbonyl-2,2-dimethylthiazolidine-4-carbothioamide With potassium hydrogencarbonate In tetrahydrofuran at 20℃; for 3h; Cooling with ice; Stage #2: With pyridine; trifluoroacetic anhydride In tetrahydrofuran Cooling with ice; | 79% |
| Stage #1: methyl 3-bromo-2-oxopropanoate; (R)-3-t-butoxycarbonyl-2,2-dimethylthiazolidine-4-carbothioamide With potassium hydrogencarbonate In tetrahydrofuran at 20℃; Stage #2: With pyridine; trifluoroacetic anhydride In ethyl acetate Cooling with ice; |
-
-
79456-34-1
5-bromo-3-(trifluoromethyl)pyridin-2-amine

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
1121058-16-9
6-Bromo-8-trifluoromethyl-imidazo[1,2-a]pyridine-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| at 50℃; for 3h; | 82% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
1379165-19-1
3-trifluoromethyl-1,2-dihydro-pyridin-2-ylamine

-
-
1206972-73-7
8-trifluoromethyl-imidazo[1,2-a]pyridine-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 60℃; for 1h; | 80% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
343-94-2
tryptamine hydochloride

-
-
1189356-88-4
methyl 1-(bromomethyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-carboxylate

| Conditions | Yield |
|---|---|
| With pyrographite In methanol for 18h; Pictet-Spengler condensation; Reflux; Inert atmosphere; | 78% |
| In methanol at 80℃; |
-
-
36727-08-9
6-hydroxybenzothiazole-2-thiocarboxamide

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
36727-10-3
dehydroluciferin methyl ester

| Conditions | Yield |
|---|---|
| In methanol 1.) 25 deg C, 21 h, 2.) reflux, 1.3 h; | 77% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
2227-79-4
benzenecarbothioamide

-
-
7113-02-2
2-phenyl-1,3-thiazole-4-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 18h; Reflux; | 77% |

| Conditions | Yield |
|---|---|
| In ethanol at 90℃; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-bromo-2-oxopropanoate; C13H22N2O4S With potassium hydrogencarbonate In tetrahydrofuran at 20℃; for 3h; Cooling with ice; Stage #2: With pyridine; trifluoroacetic anhydride In tetrahydrofuran Cooling with ice; | 75% |
| Stage #1: methyl 3-bromo-2-oxopropanoate; C13H22N2O4S With potassium hydrogencarbonate In tetrahydrofuran at 20℃; Stage #2: With pyridine; trifluoroacetic anhydride In ethyl acetate Cooling with ice; |

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

| Conditions | Yield |
|---|---|
| Stage #1: N-tert-butoxycarbonyl-O-(4-methoxybenzyl)thiothreoninamide; methyl 3-bromo-2-oxopropanoate With potassium hydrogencarbonate In 1,2-dimethoxyethane at 0℃; for 1h; Hantzsch reaction; Stage #2: With 2,6-dimethylpyridine; trifluoroacetic anhydride In 1,2-dimethoxyethane at 0℃; for 2h; Further stages.; | 74% |
-
-
110-86-1
pyridine

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
1146344-31-1
3-cyclohexylimino-2-(diisopropoxy-phosphoryloxy)-acrylic acid ethyl ester

-
-
1259393-58-2
C19H24N2O4

| Conditions | Yield |
|---|---|
| Stage #1: pyridine; methyl 3-bromo-2-oxopropanoate In acetonitrile at 60℃; for 0.5h; Stage #2: 3-cyclohexylimino-2-(diisopropoxy-phosphoryloxy)-acrylic acid ethyl ester With potassium carbonate In acetonitrile at 20℃; | 74% |

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
1421637-26-4
3-amino-4-bromo-6-(4-fluorophenyl)pyridazine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 70℃; for 17h; | 73% |
| In N,N-dimethyl-formamide at 75℃; for 4h; Temperature; | 19.9 g |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
183610-70-0
2-amino-3-trifluoromethylpyridine

-
-
1206972-73-7
8-trifluoromethyl-imidazo[1,2-a]pyridine-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 60℃; for 3h; | 72% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 75℃; for 3h; | 72% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
105-45-3
acetoacetic acid methyl ester

-
-
6141-60-2
dimethyl 2-methylfuran-3,4-dicarboxylate

| Conditions | Yield |
|---|---|
| at 50℃; Feist-Benary Furan Synthesis; | 72% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
141041-86-3, 96929-01-0, 140903-30-6
tert-butyl (S)-(1-amino-1-thioxopropan-2-yl)carbamate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-bromo-2-oxopropanoate; tert-butyl (S)-(1-amino-1-thioxopropan-2-yl)carbamate With potassium hydrogencarbonate In tetrahydrofuran at 20℃; for 3h; Cooling with ice; Stage #2: With pyridine; trifluoroacetic anhydride In tetrahydrofuran Cooling with ice; | 72% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
1610008-47-3
6-chloro-4-(trifluoromethyl)pyridazin-3-amine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 70℃; for 5h; | 70% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide at 50℃; for 1.5h; Inert atmosphere; | 65.66% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
62-55-5
thioacetamide

-
-
6436-60-8
methyl 2-methyl-1,3-thiazole-4-carboxylate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Inert atmosphere; Heating; | 65% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 65℃; for 2h; Inert atmosphere; | 65% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

-
-
108-98-5
thiophenol

-
-
115413-26-8
Methyl 2-oxo-3-phenylthiopropionate

| Conditions | Yield |
|---|---|
| With pyridine In diethyl ether at 0 - 5℃; for 0.5h; | 64% |
-
-
446273-59-2
4-bromo-6-chloro-3-pyridazineamine

-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide; N,N-dimethyl-formamide at 70℃; for 18h; | 62% |
-
-
7425-63-0
methyl 3-bromo-2-oxopropanoate

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In acetonitrile at 20℃; for 12h; Pictet-Spengler Synthesis; | 61% |
Methyl bromopyruvate Chemical Properties
Molecular Structure of Methyl bromopyruvate (7425-63-0):
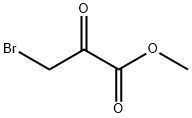
IUPAC Name: Methyl 3-bromo-2-oxopropanoate
Molecular Formula: C4H5BrO3
Molecular Weight: 180.9847 g/mol
XLogP3-AA: 0.8
H-Bond Donor: 0
H-Bond Acceptor: 3
Canonical SMILES: COC(=O)C(=O)CBr
InChI: InChI=1S/C4H5BrO3/c1-8-4(7)3(6)2-5/h2H2,1H3
InChIKey: MQONVZMIFQQQHA-UHFFFAOYSA-N
Index of Refraction: 1.467 Molar
Refractivity: 30.11 cm3
Molar Volume: 108.3 cm3
Surface Tension: 40.4 dyne/cm
Density: 1.67 g/cm3
Flash Point: 66.8 °C
Boiling Point: 186.8 °C at 760 mmHg
Enthalpy of Vaporization: 42.31 kJ/mol
Vapour Pressure: 0.65 mmHg at 25 °C
Water Solubility: mg/L at 25 °C
Refractive Index: n20/D 1.480
Storage Temp.: 2-8°C
Methyl bromopyruvate Safety Profile
Safety Information of Methyl bromopyruvate (7425-63-0):
Hazard Codes: Xi ![]()
Risk Statements: 36/37/38
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
WGK Germany: 3
F: 19
Methyl bromopyruvate Specification
Methyl bromopyruvate (7425-63-0) can be soluble in many organic solvents which is also called for Methyl 3-bromo-2-oxopropanoate ; 2-Oxo-3-bromopropionic acid methyl ester ; 3-Bromo-2-oxopropionic acid methyl ester . It can be used as pharmaceutical intermediate, pharmaceutical raw material, mainly used to manufacture Thiabendazole .
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 74257-00-4
- 7425-81-2
- 74258-86-9
- 7425-93-6
- 7426-02-0
- 74263-37-9
- 74263-38-0
- 742666-57-5
- 74266-66-3
- 74266-68-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View