-
Name
Mifepristone
- EINECS 617-559-7
- CAS No. 84371-65-3
- Article Data2
- CAS DataBase
- Density 1.18 g/cm3
- Solubility Soluble to 100 mM in DMSO and to 50 mM in Ethanol
- Melting Point 195-198 °C
- Formula C29H35NO2
- Boiling Point 628.6 °C at 760 mmHg
- Molecular Weight 429.602
- Flash Point 334 °C
- Transport Information
- Appearance pale yellow solid
- Safety 53-22-36/37/39-45
- Risk Codes 60-61
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Estra-4,9-dien-3-one,11-[4-(dimethylamino)- phenyl]-17-hydroxy-17-(1-propynyl)-,(11a,- 17a)-;Mifepristone [USAN:BAN:INN];17-beta-Hydroxy-11-beta-(4-dimethylaminophenyl-1)-17-alpha-(prop-1-ynyl)oestra-4,9-dien-3-one;Mifeprex;Mifepristonum [Latin];R 38486;RU 38486;11beta-(4-(Dimethylamino)phenyl)-17beta-hydroxy-17-(1-propynyl)estra-4,9-dien-3-on;Estra-4,9-dien-3-one, 11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(1-propynyl)-, (11b,17b)-;Prestwick_570;Mifegyne;(11beta,17beta)-11-(4-(Dimethylamino)-phenyl)-17-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one;RU-486;RU486;11beta-(4-(Dimethylamino)phenyl)-17-hydroxy-21-methyl-19-nor-17alpha-pregna-4,9-dien-20-m-3-on;RU 486-6;17-(1-propynyl)estra-4,9-dien-3-one;
- PSA 40.54000
- LogP 5.40650
Synthetic route
-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With hydrogen In methanol under 3800.26 Torr; Reagent/catalyst; Solvent; Green chemistry; stereoselective reaction; | 99% |
-
-
98-98-6
2-Picolinic acid

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With [MoI2(CO)2(CNC6H3(C6H3iPr2-2,6)2-2,6)2] In benzene-d6 at 20℃; for 0.5h; Inert atmosphere; Schlenk technique; Glovebox; | 98% |
-
-
84371-65-3
mifepristone

-
-
104004-96-8
Demethylmifepristone

| Conditions | Yield |
|---|---|
| With lithium acetate; iodine In tetrahydrofuran; methanol at 0 - 20℃; for 24h; Reagent/catalyst; Temperature; Solvent; | 97% |
| With lithium acetate; iodine In tetrahydrofuran; methanol at 20℃; | 92% |
| With iodine; calcium oxide In tetrahydrofuran; methanol at 0℃; for 1h; | 82% |
-
-
84371-65-3
mifepristone

-
-
79-04-9
chloroacetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate at 0 - 20℃; for 16h; | 92% |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
135053-66-6
methyl (3β,5β,7α,12α)-7,12-dixydroxy-3-(2-hydroxyethoxy)cholan-24-oate

-
-
84371-65-3
mifepristone

-
-
639520-78-8
methyl (3β,5β,7α,12α)-7,12-dihydroxy-3-{2-[{4-[(11β,17α)-17-hydroxy-3-oxo-17-prop-1-ynylestra-4,9-dien-11-yl]phenyl}(methyl)amino]ethoxy}cholan-24-oate

| Conditions | Yield |
|---|---|
| Stage #1: trifluoromethylsulfonic anhydride; methyl (3β,5β,7α,12α)-7,12-dixydroxy-3-(2-hydroxyethoxy)cholan-24-oate With N-ethyl-N,N-diisopropylamine In dichloromethane at -55 - -45℃; for 3.28333h; Stage #2: mifepristone In dichloromethane at -6℃; for 88h; Stage #3: With N-ethyl-N,N-diisopropylamine; sodium iodide In acetonitrile for 45h; Heating / reflux; | 82.7% |

-
-
84371-65-3
mifepristone

-
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 77% |
-
-
58574-03-1
4'-Hydroxybiphenyl-4-carboxylic acid

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 73% |
-
-
51-44-5
3,4-dichlorbenzoic acid

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16h; | 71% |
-
-
3262-89-3
triphenylboroxine

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With [Rh(OH)(cod)]2 In tetrahydrofuran at 75℃; for 12h; Inert atmosphere; diastereoselective reaction; | 69% |
-
-
128796-39-4
4-trifluoromethylphenylboronic acid

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With water; 8CO*2Br(1-)*2Mn(1+); potassium hydrogencarbonate In toluene; pentane at 90℃; for 0.25h; Sealed tube; regioselective reaction; | 68% |
-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With tetrachloromethane; potassium fluoride; 18-crown-6 ether In tetrahydrofuran at 65℃; for 10h; Sealed tube; | 67% |
-
-
84371-65-3
mifepristone

-
A
-
104004-96-8
Demethylmifepristone

-
B
-
639520-83-5
N-(4-((8S,11R,13S,14S,17S)-17-hydroxy-13-methyl-3-oxo-17-(prop-1-yn-1-yl)-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-11-yl)phenyl)-N-methylformamide

| Conditions | Yield |
|---|---|
| Stage #1: mifepristone With 1,4-diaza-bicyclo[2.2.2]octane; rose bengal; acetic acid In acetonitrile at 20℃; for 1.5h; Flow reactor; Irradiation; Stage #2: With ascorbic acid In water; acetonitrile at 20℃; for 2h; | A 65% B 22% |
-
-
84371-65-3
mifepristone

-
-
639520-83-5
N-(4-((8S,11R,13S,14S,17S)-17-hydroxy-13-methyl-3-oxo-17-(prop-1-yn-1-yl)-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-11-yl)phenyl)-N-methylformamide

| Conditions | Yield |
|---|---|
| With N-methyl-2-indolinone; tetrapropylammonium perruthennate In dichloromethane at 0℃; for 0.333333h; | 63% |
| Stage #1: mifepristone With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide In dichloromethane at -10℃; Stage #2: With sodium disulfite In dichloromethane; water at -10 - 20℃; for 0.5h; | 63% |
-
-
941-69-5
N-phenyl-maleimide

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With chloropyridinecobaloxime(III); Ru(bpy)3Cl2*6H2O In acetonitrile at 20℃; for 24h; Inert atmosphere; Irradiation; | 62% |
-
-
84371-65-3
mifepristone

-
-
446-30-0
4-chloro-2-fluorobenzoic acid

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 30h; | 61% |
-
-
639520-81-3
methyl (3β,5β,7α,12α)-7,12-dihydroxy-3-[2-(iodo)ethoxy]cholan-24-oate

-
-
84371-65-3
mifepristone

-
-
639520-78-8
methyl (3β,5β,7α,12α)-7,12-dihydroxy-3-{2-[{4-[(11β,17α)-17-hydroxy-3-oxo-17-prop-1-ynylestra-4,9-dien-11-yl]phenyl}(methyl)amino]ethoxy}cholan-24-oate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl acetamide at 80℃; for 19h; | 60.6% |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-1,2-benzodioxol-3-(1H)-one; trimethylsilylazide In acetonitrile at 20℃; Inert atmosphere; chemoselective reaction; | 54% |
-
-
84371-65-3
mifepristone

-
-
1356033-06-1
C29H33NO2

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetic acid at 20℃; for 1h; Sonication; | 41% |
-
-
84371-65-3
mifepristone

-
-
1356033-08-3
C29H32Cl3NO2

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine In acetonitrile at 20℃; for 48h; | 41% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 120℃; for 2h; Microwave irradiation; | 40% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 120℃; for 1h; Microwave irradiation; | 36% |

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In 1,4-dioxane at 80℃; for 20h; | A 4% B 35% |
-
-
84371-65-3
mifepristone

-
-
1356033-03-8
C28H33NO5

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In dichloroethane at 20℃; for 4h; | 32% |
-
-
84371-65-3
mifepristone

-
-
1356033-05-0
C28H31NO3

| Conditions | Yield |
|---|---|
| With Cumene hydroperoxide; C44H24FeN8O8 In dichloroethane at 20℃; for 6h; | 19% |
-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In dichloromethane at 20℃; for 15h; | 18% |

| Conditions | Yield |
|---|---|
| for 336h; Irradiation; photodecomposition in crystalline state; other times; | A 0.2% B 0.5% |
| for 336h; Irradiation; | A 0.2% B 0.5% |
-
-
84371-65-3
mifepristone


| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 2h; Title compound not separated from byproducts; |

-
-
84371-65-3
mifepristone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 63 percent / NMO; tetra-n-propylammonium perruthenate / CH2Cl2 / 0.33 h / 0 °C 2: 86 percent / aq. HCl / methanol / 40 h / 20 °C 3: pyridine / 2 h / 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: lithium acetate; iodine / tetrahydrofuran; methanol / 20 °C 2: triethylamine / ethyl acetate / 16 h / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
84371-65-3
mifepristone

-
-
734523-73-0
(S)-2-Amino-4-{[4-((8S,11R,13S,14S,17S)-17-hydroxy-13-methyl-3-oxo-17-prop-1-ynyl-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-11-yl)-phenyl]-methyl-carbamoyl}-butyric acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 63 percent / NMO; tetra-n-propylammonium perruthenate / CH2Cl2 / 0.33 h / 0 °C 2: 86 percent / aq. HCl / methanol / 40 h / 20 °C 3: 0.93 g / TBTU; diisopropylethylamine / dimethylformamide / 48 h / 20 °C 4: HCl / ethyl acetate / 1 h View Scheme |
Mifepristone History
In 1980, Mifepristone (CAS NO.84371-65-3) was discovered by researchers at Roussel Uclaf of France while they were studying glucocorticoid receptor antagonists.étienne-émile Baulieu recognized its anti-progesterone activities and saw its potential for the induction of a medical abortion. Clinical testing began in 1982. The drug was first licensed in France in 1988, for use in combination with a prostaglandin, under the name Mifegyne. After license approval but before market release, Roussel Uclaf announced it would abandon distribution of the drug, bowing to pressure from pro-life groups and the threat of a boycott. However, two days later, the French government, part owner of Roussel Uclaf, intervened, leading to the resumption of production and distribution of RU-486.
Mifepristone Specification
1. Introduction of Mifepristone
Mifepristone is one kind of pale yellow solid. The IUPAC Name of this chemical is (8S,11R,13S,14S,17S)-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one. Mifepristone belongs to Steroids;Hormone;Acetylenes;Biochemistry;Functionalized Acetylenes;Hydroxyketosteroids;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Intracellular receptor;Nuclear Receptors. Mifepristone is soluble in organic solvents but poorly soluble in water.
The Classification Code of this chemical is Abortifacient Agents; Abortifacient agents, steroidal; Contraception; Contraceptive Agents; Contraceptive Agents, Female; Contraceptives, Oral; Contraceptives, Postcoital; Contraceptives, oral, synthetic; Contraceptives, postcoital, synthetic; Hormone; Hormone antagonists; Hormones, Hormone Substitutes, and Hormone Antagonists; Human Data; Induction of abortion; Luteolytic agents; Menstruation-inducing agents; Reproductive Control Agents; Reproductive Effect; Treatment of gynecological disorders [progesterone antagonist].
2. Properties of Mifepristone
Physical properties about Mifepristone are:
(1)Index of Refraction: 1.623; (2)Molar Refractivity: 127.71 cm3; (3)Molar Volume: 362 cm3; (4)Polarizability: 50.62×10-24 cm3; (5)Surface Tension: 54 dyne/cm; (6)Density: 1.18 g/cm3; (7)Flash Point: 334 °C; (8)Enthalpy of Vaporization: 97.77 kJ/mol; (9)Melting Point: 195-198°C; (10)Boiling Point: 628.6 °C at 760 mmHg; (11)Vapour Pressure: 1.14E-16 mmHg at 25°C; (12)XLogP3-AA: 3.8; (13)H-Bond Donor: 1; (14)H-Bond Acceptor: 3.
3. Structure Descriptors of Mifepristone
(1)Canonical SMILES: CC#CC1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O
(2)InChIKey: VKHAHZOOUSRJNA-GCNJZUOMSA-N
(3)Isomeric SMILES: CC#C[C@@]1(CC[C@@H]2[C@@]1(C[C@@H](C3=C4CCC(=O)C=C4CC[C@@H]23)C5=CC=C(C=C5)N(C)C)C)O
(4)InChI: InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1
(5)Smiles: C1=2[C@H]([C@H]3[C@]([C@@](C#CC)(O)CC3)(C)C[C@@H]1c1ccc(N(C)C)cc1)CCC=1C2CCC(C1)=O
4. Safety information of Mifepristone
Hazard Codes:  T
T
Risk Statements: 60-61
S60:This material and its container must be disposed of as hazardous waste.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
Safety Statements: 53-22-36/37/39-45
S53:Avoid exposure - obtain special instructions before use.
S22:Do not breathe dust.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
WGK Germany: 3
RTECS: KG2955000
5. Uses of Mifepristone
Mifepristone (CAS NO.84371-65-3) is a synthetic steroid compound used as a pharmaceutical. It is used as an abortifacient in the first two months of pregnancy, and in smaller doses as an emergency contraceptive. Other medical applications of mifepristone that have been studied in Phase II clinical trials include regular long-term use as an oral contraceptive, and treatment of: uterine fibroids, endometriosis, major depression with psychotic features, glaucoma, meningiomas, breast cancer, ovarian cancer, prostate cancer, and some types of Cushing's syndrome.
6. Production of Mifepristone
(1)Steroidal 4,9 estrogen diene-3 ,17-dione 3 ketal compound (I) as raw material and propynyl magnesium bromide Grignard reagent, at 17 for introduction of the propargyl group, to obtain the compound (II). Followed by five ethylenically for selective epoxidation to give compound (III). Containing copper bromide - dimethyl sulfide complex in tetrahydrofuran, and dimethylaminophenyl magnesium bromide in 11 introduces dimethylaminophenyl to give the compound (IV). Finally, in methanol, by hydrolysis with hydrochloric acid and dehydrated to obtain a mifepristone. Yield is 21.5%.
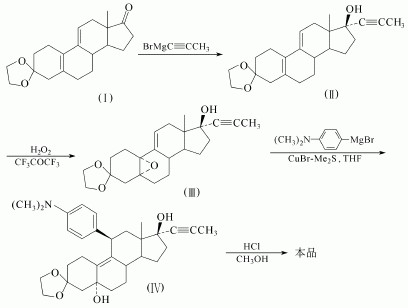
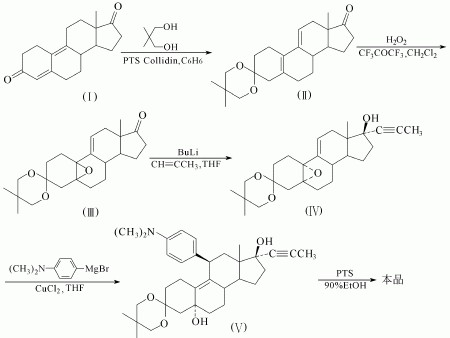
(2) Another way to get the Mifepristone is showed as follows:
Related Products
- Mifepristone
- 84376-20-5
- 84377-97-9
- 84379-13-5
- 84380-01-8
- 84380-10-9
- 84381-54-4
- 84385-23-9
- 84387-62-2
- 84387-89-3
- 84-38-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View