-
Name
N-Ethylmethylamine
- EINECS 210-862-1
- CAS No. 624-78-2
- Article Data60
- CAS DataBase
- Density 0.68 g/cm3
- Solubility
- Melting Point -70.99°C (estimate)
- Formula C3H9N
- Boiling Point 36.7 °C at 760 mmHg
- Molecular Weight 59.1112
- Flash Point <?30°F
- Transport Information UN 2733 3/PG 1
- Appearance clear colorless to light yellow liquid
- Safety 26-36/37/39-45
- Risk Codes 11-20/21/22-35
-
Molecular Structure
-
Hazard Symbols
 F;
F;  C
C
- Synonyms N-methyl-aminoethane;Methylaminoethane;2-MAE;N-METHYLETHYLAMINE;Methylethylamine;n-methyl-ethanamin;(CH3)(C2H5)NH;Ethylmethylamine;methyl-N-ethylamine;N-ethyl-N-methylamine;
- PSA 12.03000
- LogP 0.61660
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; sodium hydroxide; Raney nickel at 65 - 67℃; under 22502.3 Torr; for 4.3h; Product distribution / selectivity; Autoclave; Industry scale; | 90% |
| With potassium hydroxide; dipotassium hydrogenphosphate; phosphoric acid at 10 - 12℃; for 4h; electrochemical reductive amination, lead cathode, current density 0.05 A/cm2, pH 12; Yield given; | |
| With imine reductase from Streptomyces ipomoeae; NADPH In water at 30℃; for 0.166667h; pH=9.5; Catalytic behavior; Reagent/catalyst; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In ethanol | 80% |
-
-
3710-84-7
N-ethyl-N-hydroxy-ethanamine

-
A
-
50-00-0
formaldehyd

-
B
-
624-78-2
N-Ethylmethylamine

-
C
-
75-04-7
ethylamine

-
D
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In toluene at 18 - 20℃; Mechanism; Product distribution; object of study: Stieglitz rearrangement (prototype); | A n/a B 1.4% C 76% D n/a |

-
-
6852-54-6
N-(benzylidene)ethylamine

-
-
5187-82-6
(ethoxycarbonylmethyl)dimethylsulfonium bromide

-
A
-
624-78-2
N-Ethylmethylamine

-
B
-
94117-87-0
1-methylthio-3-phenyl-1,2-cyclopropanedicarboxylic acid diethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In tetrahydrofuran at 20℃; for 4h; | A n/a B 50% |

-
-
110-86-1
pyridine

-
-
616-39-7
N,N-diethylnmethylamine

-
-
94-36-0
dibenzoyl peroxide

-
A
-
50-00-0
formaldehyd

-
B
-
624-78-2
N-Ethylmethylamine

-
C
-
75-07-0
acetaldehyde

-
D
-
109-89-7
diethylamine

-
-
60-29-7
diethyl ether

-
-
6154-14-9
N-chloromethylamine

-
-
557-20-0
diethylzinc

-
A
-
624-78-2
N-Ethylmethylamine

-
B
-
74-89-5
methylamine


| Conditions | Yield |
|---|---|
| With Petroleum ether |
-
-
10595-95-6
N-nitrosomethylethylamine

-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; acetic acid at 65 - 75℃; under 2206.5 Torr; durch Hydrogenolyse; |

| Conditions | Yield |
|---|---|
| With ethanol at 100℃; |
-
-
4186-68-9
1-Ethyl-1-methylpyrrolidinium iodide

-
-
141-43-5
ethanolamine

-
A
-
120-94-5
1-Methylpyrrolidine

-
B
-
7335-06-0
1-ethylpyrrolidine

-
C
-
2955-88-6
1-pyrrolidineethanol

-
D
-
624-78-2
N-Ethylmethylamine


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; 3,6,9-trioxaundecane | |
| With hydrogen; nickel at 180 - 190℃; |
-
-
108-24-7
acetic anhydride

-
-
596-58-7, 70630-96-5, 70630-97-6, 70680-98-7
(RS)-disinomenine

-
A
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| at 180℃; isomer(ic) I; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide at 180℃; |

| Conditions | Yield |
|---|---|
| Kochen des endstandenen Jodmethylats mit Alkohol; |

| Conditions | Yield |
|---|---|
| (i) aq. NaOH, HSO4, (ii) HCl; Multistep reaction; |

| Conditions | Yield |
|---|---|
| In ethanol at 100℃; under 3677.5 Torr; |
-
-
7779-27-3
1,3,5-triethyl-1,3,5-triazacyclohexane

-
-
91076-68-5
mono-trimethylsilylphosphite

-
A
-
598-56-1
N,N-dimethyl-ethanamine

-
B
-
624-78-2
N-Ethylmethylamine


| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 100℃; Rate constant; Mechanism; |
-
-
519-73-3
triphenylmethane

-
-
57018-31-2
lithium ethylmethyl amide

-
A
-
624-78-2
N-Ethylmethylamine

-
B
-
733-90-4
trityllithium

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 30℃; Equilibrium constant; |


-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| With water; hydroxide |
-
-
51-75-2
nitrogen mustard

-
A
-
64-17-5
ethanol

-
B
-
624-78-2
N-Ethylmethylamine

-
C
-
616-39-7
N,N-diethylnmethylamine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; aluminum nickel In methanol Product distribution; degradation under various conditions with preparation of nonmutagenic reaction mixtures of products; |

-
-
107-12-0
propiononitrile

-
A
-
624-78-2
N-Ethylmethylamine

-
B
-
627-35-0
methyl-n-propylamine

-
C
-
75-04-7
ethylamine

-
D
-
123-38-6
propionaldehyde

| Conditions | Yield |
|---|---|
| weiteres Produkt:Ammoniak; |

-
-
107-12-0
propiononitrile

-
A
-
624-78-2
N-Ethylmethylamine

-
B
-
627-35-0
methyl-n-propylamine

-
C
-
75-04-7
ethylamine

-
D
-
123-38-6
propionaldehyde

| Conditions | Yield |
|---|---|
| weiteres Produkt:Ammoniak; |

-
-
109-90-0
ethyl isocyanate

-
A
-
624-78-2
N-Ethylmethylamine

-
B
-
7664-41-7
ammonia

-
C
-
75-04-7
ethylamine

-
D
-
109-89-7
diethylamine

| Conditions | Yield |
|---|---|
| at 180 - 190℃; Produkt 5: Triaethylamin; |


-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc zuletzt bei Siedetemperatur; |

| Conditions | Yield |
|---|---|
| In water Heating; | 100% |
-
-
624-78-2
N-Ethylmethylamine

-
-
287721-05-5
4-[phenyl(endo-8-methyl-8-azabicyclo[3.2.1]octan-3-yl)amino]benzoic acid

-
-
287720-97-2
N-ethyl-N-methyl-4-[(8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl)-phenyl-amino]-benzamide

| Conditions | Yield |
|---|---|
| With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate Acylation; | 100% |

-
-
624-78-2
N-Ethylmethylamine

-
-
954424-88-5
ethyl-methyl-{3-[3-(4-methyl-piperidin-1-yl)-4-nitro-phenyl]-prop-2-ynyl}-amine

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.333333h; Heating / reflux; | 100% |
-
-
945001-04-7
3-[3-(phenylsulfonyl)-1H-indol-4-yl]propyl 4-methylbenzenesulfonate

-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| Stage #1: 3-[3-(phenylsulfonyl)-1H-indol-4-yl]propyl 4-methylbenzenesulfonate; N-Ethylmethylamine In tetrahydrofuran at 65℃; for 16h; Stage #2: With sodium hydroxide; water In tetrahydrofuran | 100% |
| Stage #1: 3-[3-(phenylsulfonyl)-1H-indol-4-yl]propyl 4-methylbenzenesulfonate; N-Ethylmethylamine In tetrahydrofuran at 65℃; for 16h; Stage #2: With hydrogenchloride In ethanol | 69% |
-
-
624-78-2
N-Ethylmethylamine

-
-
1263868-77-4
2,4-dichloro-8-phenyl-7,8-dihydro-5H-pyrano[4,3-d]pyrimidine

-
-
1263868-83-2
2-chloro-N-ethyl-N-methyl-8-phenyl-7,8-dihydro-5H-pyrano[4,3-d]pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |
| In methanol at 20℃; for 1h; | 100% |
-
-
624-78-2
N-Ethylmethylamine

-
-
1263870-09-2
2,4-dichloro-8-(4-fluorophenyl)-5,6,7,8-tetrahydroquinazoline

-
-
1263870-13-8
2-chloro-N-ethyl-8-(4-fluorophenyl)-N-methyl-5,6,7,8-tetrahydroquinazolin-4-amine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.5h; | 100% |
| In methanol at 20℃; for 0.5h; | 100% |
-
-
624-78-2
N-Ethylmethylamine

-
-
1263868-24-1
2,4-dichloro-7-phenyl-6,7-dihydro-5H-cyclopenta[d]pyrimidine

-
-
1263868-27-4
2-chloro-N-ethyl-N-methyl-7-phenyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-amine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |
| In methanol at 20℃; for 1h; | 100% |
-
-
624-78-2
N-Ethylmethylamine

-
-
29769-77-5
3-methoxy-4-methyl-3-cyclobutene-1,2-dione

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: N-Ethylmethylamine With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.25h; Stage #2: carbon disulfide In tetrahydrofuran; hexane at 20℃; for 3h; | 100% |
-
-
13139-16-7
N-(tert-butyloxycarbonyl)-L-isoleucine

-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate In triethylamine; N,N-dimethyl-formamide Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-(2-ethyl-3-chloropyridyl-4-oxy)-2,5-xylidine; trimethyl orthoformate With toluene-4-sulfonic acid for 3h; Heating / reflux; Stage #2: N-Ethylmethylamine In dichloromethane at 20℃; for 16h; | 99% |

-
-
624-78-2
N-Ethylmethylamine

-
-
1160490-11-8
methyl 2-(methylthio)benzo[d]oxazole-6-carboxylate

-
-
1160490-12-9
C12H14N2O3

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 70℃; | 99% |
| In tetrahydrofuran at 70℃; |

| Conditions | Yield |
|---|---|
| With oxygen In isopropyl alcohol at 50℃; under 1275.13 Torr; for 2h; Autoclave; | 98.6% |
-
-
624-78-2
N-Ethylmethylamine

-
-
36260-79-4
N,N-chloromethylethylamine

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide under 0.004 Torr; Ambient temperature; stored at t<-20 deg C; | 98% |
| With N-chloro-succinimide under 1E-05 Torr; Ambient temperature; Yield given; | |
| With sodium hypochlorite; sodium perchlorate In water at 24.85℃; Kinetics; Further Variations:; Temperatures; Chlorination; |
-
-
624-78-2
N-Ethylmethylamine

-
-
1711-05-3
m-anisoyl chloride

-
-
207558-42-7
N-ethyl-3-methoxy-N-methylbenzamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 2h; | 98% |
-
-
624-78-2
N-Ethylmethylamine

-
-
942035-74-7
1-(6-bromo-3,4-dihydroquinolin-1(2H)-yl)-2-chloroethanone

-
-
1063408-94-5
1-(6-bromo-3,4-dihydroquinolin-1(2H)-yl)-2-(ethyl(methyl)amino)ethanone

| Conditions | Yield |
|---|---|
| With potassium iodide In tetrahydrofuran at 20 - 65℃; for 3h; | 98% |
| With potassium iodide In tetrahydrofuran at 20 - 65℃; for 3h; Inert atmosphere; | 98% |
-
-
624-78-2
N-Ethylmethylamine

-
-
2816-54-8
triisopropylchlorogermane

-
-
1147551-48-1
ethylmethylaminotriisopropylgermane

| Conditions | Yield |
|---|---|
| Stage #1: N-Ethylmethylamine With n-butyllithium In diethyl ether at 0℃; for 2h; Cooling with ice; Stage #2: triisopropylchlorogermane In diethyl ether at 0 - 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 60℃; for 18h; | 98% |
| With triethylamine In ethanol Reflux; | 0.93 g |
-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 25℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With triethyl phosphite In toluene at 50℃; for 12h; | 98% |

-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 12h; | 97% |
-
-
945000-91-9
2-[3-(phenylsulfonyl)-1H-indol-4-yl]ethyl 4-methylbenzenesulfonate

-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| Stage #1: 2-[3-(phenylsulfonyl)-1H-indol-4-yl]ethyl 4-methylbenzenesulfonate; N-Ethylmethylamine In tetrahydrofuran at 71℃; for 24h; Stage #2: With ammonia In ethanol; dichloromethane Stage #3: With hydrogenchloride; water In diethyl ether | 97% |
-
-
624-78-2
N-Ethylmethylamine

-
-
1253768-91-0
(Z)-3-(hydroxy-phenyl-methylene)-2-oxo-2,3-dihydro-1H-indole-6-carboxylic acid

-
-
1253768-93-2
(Z)-3-(hydroxy-phenyl-methylene)-2-oxo-2,3-dihydro-1H-indole-6-carboxylic acid N-methyl-N-ethylamide

| Conditions | Yield |
|---|---|
| Stage #1: (Z)-3-(hydroxy-phenyl-methylene)-2-oxo-2,3-dihydro-1H-indole-6-carboxylic acid With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: N-Ethylmethylamine In N,N-dimethyl-formamide at 20℃; for 24h; | 97% |
-
-
624-78-2
N-Ethylmethylamine

-
-
1144520-57-9
ethyl 2-chloro-5-methyloxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| at 20℃; for 13.5h; | 97% |
-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 96.7% |
-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| In methanol at 65℃; Inert atmosphere; Darkness; | 96.3% |

-
-
624-78-2
N-Ethylmethylamine

-
-
745827-35-4
1-ethyl-3-[2-(3-isopropyl-[1,2,4]triazolo[4,3-a]pyridin-6-ylsulfanyl)-benzyl]-1-methyl-urea

| Conditions | Yield |
|---|---|
| Stage #1: [2-(3-isopropyl-[1,2,4]triazolo[4,3-a]pyridin-6-ylsulfanyl)-benzyl]-carbamic acid phenyl ester; N-Ethylmethylamine In dimethyl sulfoxide at 20℃; for 0.5h; Stage #2: In water | 96% |

-
-
624-78-2
N-Ethylmethylamine

| Conditions | Yield |
|---|---|
| With cesium fluoride In dimethyl sulfoxide at 120℃; for 12h; | 96% |

| Conditions | Yield |
|---|---|
| With C18H17N3Ni In toluene at 75℃; for 6h; | 96% |
| With C17H15N3Pd In toluene at 75℃; for 6h; | 93% |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 5h; | 96% |
N-Ethylmethylamine Specification
The N-Ethylmethylamine, with the CAS registry number 624-78-2 and EINECS registry number 210-862-1, has the systematic name and IUPAC name of N-methylethanamine. And the molecular formula of this chemical is C3H9N. It is a kind of clear colorless to light yellow liquid which is also hygroscopic. And it should be stored at 0-6°C.
The physical properties of N-Ethylmethylamine are as following: (1)ACD/LogP: 0.10; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3; (4)ACD/LogD (pH 7.4): -2.81; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.367; (14)Molar Refractivity: 19.54 cm3; (15)Molar Volume: 86.8 cm3; (16)Polarizability: 7.74×10-24cm3; (17)Surface Tension: 18.2 dyne/cm; (18)Density: 0.68 g/cm3; (19)Enthalpy of Vaporization: 28.16 kJ/mol; (20)Boiling Point: 36.7 °C at 760 mmHg; (21)Vapour Pressure: 488 mmHg at 25°C.
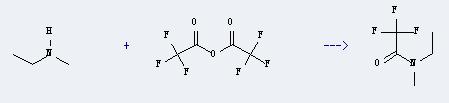
Uses of N-Ethylmethylamine: It can react with trifluoroacetic acid anhydride to produce N-ethyl-N-methyltrifluoroacetamide. This reaction will need solvent diethyl ether.
You should be cautious while dealing with this chemical. It is a kind of highly flammable chemical ehich may also cause severe burns. And it is also harmful by inhalation, in contact with skin and if swallowed. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible); Keep away from sources of ignition - No smoking.
You can still convert the following datas into molecular structure:
(1)SMILES: N(CC)C
(2)InChI: InChI=1/C3H9N/c1-3-4-2/h4H,3H2,1-2H3
(3)InChIKey: LIWAQLJGPBVORC-UHFFFAOYAE
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View