-
Name
N,N-Dimethyloctylamine
- EINECS 230-939-3
- CAS No. 7378-99-6
- Article Data58
- CAS DataBase
- Density 0.783 g/cm3
- Solubility slightly soluble in water
- Melting Point -57 °C
- Formula C10H23N
- Boiling Point 191.643 °C at 760 mmHg
- Molecular Weight 157.299
- Flash Point 65 °C
- Transport Information UN 2922 8/PG 3
- Appearance clear liquid
- Safety 26-45-36/37/39-27
- Risk Codes 25-37/38-41-34-36
-
Molecular Structure
-
Hazard Symbols
 T,
T, C
C
- Synonyms Octylamine,N,N-dimethyl- (6CI,7CI,8CI);1-(Dimethylamino)octane;Adma 8;Dimethyl-n-octylamine;Dimethyloctylamine;Farmin DM 0898;Farmin DM 08P;N,N-Dimethyl-1-octanamine;N,N-Dimethyl-n-octylamine;N-Octyl-N,N-dimethylamine;N-Octyldimethylamine;NSC 63928;Octyldimethylamine;
- PSA 3.24000
- LogP 2.90850
Synthetic route

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| With triethyl borane; phenylsilane; sodium hydroxide In tetrahydrofuran; tert-butyl methyl ether at 20℃; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 94% |
| With triethyl borane; phenylsilane; sodium hydroxide In tetrahydrofuran; tert-butyl methyl ether at 20℃; for 48h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | 94% |
| With water; sodium; sodium chloride In hexane; mineral oil at 0℃; for 3h; Inert atmosphere; | 37% |
| With diethyl ether; diisobutylaluminium hydride | |
| With hydrogen In 1,2-dimethoxyethane at 70℃; under 22502.3 Torr; for 18h; Autoclave; Molecular sieve; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene for 72h; Autoclave; | 90% |

| Conditions | Yield |
|---|---|
| With hydrogen In water; tert-butyl alcohol at 120℃; under 30003 Torr; for 24h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 100℃; for 24h; | 85.2% |

| Conditions | Yield |
|---|---|
| chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium (II) at 100℃; | 100% |
| With 5percent silver supported on titanium oxide at 25℃; for 6h; Inert atmosphere; UV-irradiation; Sealed tube; | 99% |
| With [Cp*Ir(2-(1H-benzo[d]imidazol-2-yl)-1H-benzo[d]imidazole)Cl][Cl]; caesium carbonate at 120℃; for 12h; Schlenk technique; | 93% |
-
-
67-56-1
methanol

-
-
111-86-4
n-Octylamine

-
A
-
7378-99-6
N,N-dimethyloctanamide

-
B
-
2439-54-5
N-methyl-N-octylamine

| Conditions | Yield |
|---|---|
| With γ-Al2O3 In gaseous matrix at 280℃; Yield given; Yields of byproduct given; | |
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)amino]ruthenium(II); hydrogen at 110℃; under 30003 Torr; for 24h; Glovebox; | A 7 %Spectr. B n/a |
| With platinum on carbon; sodium hydroxide at 150℃; under 750.075 Torr; for 36h; Reagent/catalyst; Inert atmosphere; Autoclave; | A 64 %Chromat. B 23 %Chromat. |


| Conditions | Yield |
|---|---|
| With hydrogen; fused iron catalyst at 250 - 265℃; under 15001.2 - 22501.8 Torr; Mechanism; other alcohols; | 87% |
| fused iron catalyst at 250 - 265℃; under 15001.2 - 22501.8 Torr; | 87% |
| With C19H32Cl2IrN2; potassium carbonate In water at 120℃; for 40h; Reagent/catalyst; Time; Temperature; Sealed tube; Green chemistry; | 83% |

| Conditions | Yield |
|---|---|
| Stage #1: octyldimethylamine oxide With N,N-Dimethylthiocarbamoyl chloride In dichloromethane at 20℃; for 2h; Stage #2: In acetonitrile for 3h; Reflux; | 92% |
| With trimethylacetic formic anhydride In chloroform 1.) 0 deg C, 10 min, 2.) 0 deg C to r.t., 30 min; Yield given; |
-
-
67-56-1
methanol

-
-
111-86-4
n-Octylamine

-
A
-
7378-99-6
N,N-dimethyloctanamide

-
B
-
2439-54-5
N-methyl-N-octylamine

| Conditions | Yield |
|---|---|
| With platinum on carbon; sodium hydroxide at 150℃; under 750.075 Torr; for 36h; Reagent/catalyst; Inert atmosphere; Autoclave; | A 47 %Chromat. B 29 %Chromat. C 12 %Chromat. |

-
-
67-56-1
methanol

-
-
111-86-4
n-Octylamine

-
A
-
7378-99-6
N,N-dimethyloctanamide

-
B
-
2439-54-5
N-methyl-N-octylamine

-
C
-
1120-48-5
n-dioctylamine

| Conditions | Yield |
|---|---|
| With rhodium contaminated with carbon; sodium hydroxide at 150℃; under 750.075 Torr; for 36h; Inert atmosphere; Autoclave; | A 62 %Chromat. B 11 %Chromat. C 5 %Chromat. |

-
-
67-56-1
methanol

-
-
111-86-4
n-Octylamine

-
A
-
7378-99-6
N,N-dimethyloctanamide

-
B
-
2439-54-5
N-methyl-N-octylamine

-
C
-
4455-26-9
methyldioctylamine

| Conditions | Yield |
|---|---|
| With platinum on zirconia; sodium hydroxide at 150℃; under 750.075 Torr; for 36h; Reagent/catalyst; Inert atmosphere; Autoclave; | A 18 %Chromat. B 35 %Chromat. C 20 %Chromat. |

-
-
67-56-1
methanol

-
-
111-86-4
n-Octylamine

-
A
-
7378-99-6
N,N-dimethyloctanamide

-
B
-
2439-54-5
N-methyl-N-octylamine

-
C
-
1120-48-5
n-dioctylamine

| Conditions | Yield |
|---|---|
| With platinum-doped magnesium oxide; sodium hydroxide at 150℃; under 750.075 Torr; for 36h; Reagent/catalyst; Inert atmosphere; Autoclave; | A 56 %Chromat. B 18 %Chromat. C 10 %Chromat. D 12 %Chromat. |

-
-
67-56-1
methanol

-
-
111-86-4
n-Octylamine

-
A
-
7378-99-6
N,N-dimethyloctanamide

-
B
-
2439-54-5
N-methyl-N-octylamine

-
C
-
1120-48-5
n-dioctylamine

-
D
-
4455-26-9
methyldioctylamine

| Conditions | Yield |
|---|---|
| With iridium on carbon; sodium hydroxide at 150℃; under 750.075 Torr; for 36h; Reagent/catalyst; Inert atmosphere; Autoclave; | A 55 %Chromat. B 14 %Chromat. C 6 %Chromat. D 12 %Chromat. |


| Conditions | Yield |
|---|---|
| With tris(triphenylphosphine)ruthenium(II) chloride In tetrahydrofuran at 180℃; for 7h; | A 11 % Chromat. B 85% |

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In hexane; water at 50℃; for 5h; | 98% |

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 0.5h; Heating; | 82% |
-
-
463-11-6
1-Fluoro-octane

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
A
-
111-85-3
1-Chlorooctane

-
B
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| With phenylmagnesium chloride In tetrahydrofuran at 80℃; for 24h; Inert atmosphere; | A 22 %Chromat. B n/a |

-
-
67-56-1
methanol

-
-
111-86-4
n-Octylamine

-
A
-
7378-99-6
N,N-dimethyloctanamide

-
B
-
4455-26-9
methyldioctylamine

| Conditions | Yield |
|---|---|
| With tris(triphenylphosphine)ruthenium(II) chloride at 180℃; for 7h; | A 12 % Chromat. B 75% |


| Conditions | Yield |
|---|---|
| With acetylacetonatodicarbonylrhodium(l); dodecacarbonyl-triangulo-triruthenium; water; triphenylphosphine In N,N-dimethyl-formamide at 170℃; for 6h; Autoclave; Green chemistry; |

-
-
1118-92-9
N,N-dimethyloctamide

-
A
-
111-87-5
octanol

-
B
-
7378-99-6
N,N-dimethyloctanamide

-
C
-
124-40-3
dimethyl amine

| Conditions | Yield |
|---|---|
| With tris(2,4-pentanedionato)ruthenium(III); ytterbium(III) trifluoromethanesulfonate nonohydrate; hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In tetrahydrofuran at 150℃; under 3750.38 Torr; for 15h; Autoclave; | A n/a B 11 %Chromat. C n/a |


| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; water at 60℃; for 12h; Temperature; Time; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With ethanol at 100℃; | |
| With PS-triphenylphosphine; di-isopropyl azodicarboxylate In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: 82 percent / LiAlH4 / tetrahydrofuran / 0.5 h / Heating View Scheme |

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 0.5h; Heating; Yield given; |

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 0.5h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| In water at 140 - 150℃; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 20℃; for 2h; |
-
-
111-85-3
1-Chlorooctane

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
111-65-9
octane

-
B
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| With bis(cyclopentadienyl)titanium dichloride; sodium tetrahydroborate 1.) 96 deg C, 1 h, 2.) 40 deg C, 7 h; Yield given. Yields of byproduct given; |

-
-
1118-92-9
N,N-dimethyloctamide

-
A
-
111-86-4
n-Octylamine

-
B
-
111-65-9
octane

-
C
-
111-87-5
octanol

-
D
-
7378-99-6
N,N-dimethyloctanamide

-
E
-
124-07-2
Octanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; Cu-Cr at 260 - 300℃; under 760 Torr; Product distribution; | A 3.9 % Chromat. B 1.8 % Chromat. C 2.9 % Chromat. D 91.8 % Chromat. E 1.1 % Chromat. |

-
-
42976-83-0
1-bromooct-4-ene

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: benzene 2: (hydrogenation) View Scheme |
-
-
7378-99-6
N,N-dimethyloctanamide

-
-
77382-63-9
(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl 2-bromoacetate

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Reflux; | 99% |
-
-
26127-08-2
(1R,2S,5R)-1-(chloromethoxy)-2-isopropyl-5-methylcyclohexane

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| In hexane at 20℃; for 0.5h; Menschutkin reaction; | 98.5% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; benzonitrile; Mg-Al-O-t-Bu HT (Catalyst B) In methanol; water at 65℃; for 0.5h; Product distribution / selectivity; | 98% |
| With aluminum oxide; Oxone In water at 80℃; Product distribution; Further Variations:; Reagents; Oxidation; | 96% |
| With dihydrogen peroxide; sodium dodecylbenzenesulfonate; layered double hydroxide WO4(2-) at 20℃; for 1h; | 95% |
| With dihydrogen peroxide In methanol; water | 78% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 82℃; for 48h; Menshutkin Reaction; | 98% |
| In ethanol Reflux; | 75% |
-
-
7378-99-6
N,N-dimethyloctanamide

-
-
49791-06-2
1-chloromethoxy-heptane

| Conditions | Yield |
|---|---|
| In n-heptane for 0.166667h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| In n-heptane for 0.166667h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| In ethyl acetate at 55℃; for 3h; | 95% |
-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Reflux; | 95% |
-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| In acetonitrile for 4h; Reflux; | 95% |
-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| In acetonitrile at 85℃; for 24h; Sealed tube; High pressure; | 95% |
-
-
939791-33-0
1-(bromomethyl)-4-(prop-1-en-2-yl)cyclohexene

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| In benzene at 20℃; | 95% |
-
-
19416-65-0
1-chloromethoxy-pentane

-
-
7378-99-6
N,N-dimethyloctanamide

| Conditions | Yield |
|---|---|
| In n-heptane for 0.166667h; Heating; | 94% |
-
-
7378-99-6
N,N-dimethyloctanamide

-
-
24566-91-4
1-chloromethoxy-nonane

| Conditions | Yield |
|---|---|
| In n-heptane for 0.166667h; Heating; | 94% |
-
-
7378-99-6
N,N-dimethyloctanamide

-
-
24566-92-5
1-chloromethoxy-decane

| Conditions | Yield |
|---|---|
| In n-heptane for 0.166667h; Heating; | 94% |
-
-
7378-99-6
N,N-dimethyloctanamide

-
-
58567-17-2
chloromethoxy-cyclooctane

| Conditions | Yield |
|---|---|
| In n-heptane for 0.166667h; Heating; | 94% |
N,N-Dimethyloctylamine Specification
The N,N-Dimethyloctylamine, with the CAS registry number 7378-99-6, is also known as Octyldimethylamine. Its EINECS registry number is 230-939-3. This chemical's molecular formula is C10H23N and molecular weight is 157.30. What's more, both its IUPAC name and systematic name are the same which is called N,N-Dimethyloctan-1-amine. It should be stored in a cool, dry and well-ventilated place.
Physical properties about N,N-Dimethyloctylamine are: (1)ACD/LogP: 3.685; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.61; (4)ACD/LogD (pH 7.4): 1.31; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.58; (7)ACD/KOC (pH 5.5): 2.02; (8)ACD/KOC (pH 7.4): 10.20; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 7; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.432; (14)Molar Refractivity: 52.099 cm3; (15)Molar Volume: 200.856 cm3; (16)Polarizability: 20.654×10-24cm3; (17)Surface Tension: 26.493 dyne/cm; (18)Density: 0.783 g/cm3; (19)Flash Point: 65 °C; (20)Enthalpy of Vaporization: 42.784 kJ/mol; (21)Boiling Point: 191.643 °C at 760 mmHg; (22)Vapour Pressure: 0.5090 mmHg at 25°C.
Preparation of N,N-Dimethyloctylamine: this chemical can be prepared by methanol with octylamine. This reaction needs reagent RuCl2(PPh3)3 at temperature of 180 °C. The reaction time is 7 hours. The yield is 90 %.

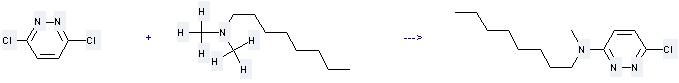
Uses of N,N-Dimethyloctylamine: (1) it is used as an intermediate in the manufacture of quaternary ammonium compounds; (2) it is used to produce other chemicals. For example, it can react with 3,6-dichloro-pyridazine to get (6-chloro-pyridazin-3-yl)-methyl-octyl-amine. The reaction occurs with solvent tetrahydrofuran at temperature of 100 °C for 4 days. The yield is 38 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin or other mucous membranes and may destroy living tissue on contacting. What's more, it may cause burns. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And you must take off immediately all contaminated clothing. In case of contacting with eyes or skin, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: N(CCCCCCCC)(C)C
(2) InChI: InChI=1S/C10H23N/c1-4-5-6-7-8-9-10-11(2)3/h4-10H2,1-3H3
(3) InChIKey: UQKAOOAFEFCDGT-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 162mg/kg (162mg/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Technical Information Service. Vol. OTS0573838, |
Related Products
- N-[(10-Oxido-9,10-dihydro-9-oxa-10-phosphaphenanthrene)methyl]-1,3,5-triazine-2,4,6-triamine
- N10-(Trifluoroacetyl)pteroic acid
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-3-amine
- N-[1,1'-Biphenyl]-4-yl-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide
- N'-[(1,1-Dimethylethoxy)carbonyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N'-methyl-L-lysine
- N1,1-Diphenyl-1,2-ethanediamine
- N-(1,2-Dimethylpropyl)-2-pyridinamine
- N<sup xmlns="">1</sup>-(3,4-DIMETHYL-5-ISOXAZOLYL)SULFANIL-AMIDE LITHIUM SALT
- 73790-05-3
- 73790-06-4
- 73790-28-0
- 73790-48-4
- 7379-12-6
- 73791-47-6
- 73791-65-8
- 73792-22-0
- 7379-35-3
- 7379-51-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View