-
Name
Nilvadipine
- EINECS 805-887-5
- CAS No. 75530-68-6
- Article Data11
- CAS DataBase
- Density 1.32 g/cm3
- Solubility
- Melting Point 148-150 °C
- Formula C19H19N3O6
- Boiling Point 526.7 °C at 760 mmHg
- Molecular Weight 385.376
- Flash Point 272.3 °C
- Transport Information
- Appearance yellow prisms
- Safety
- Risk Codes 22
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms CL 287389;Escor;FK 235;FR 34235;Nivadil;Nivadip;SKF 102362;dl-Nilvadipine;
- PSA 134.24000
- LogP 3.30988
Synthetic route
-
-
1452166-70-9
isopropyl 2-hydroxyiminomethyl-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-5-carboxylate

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| With acetic anhydride; potassium carbonate In dimethyl sulfoxide at 10 - 50℃; for 5h; | 85.1% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
A
-
75530-68-6
nilvadipine

-
B
-
145220-80-0
isopropyl 2-carbamoyl-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-1, 4-dihydropyridine-5-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogenchloride 1.) AcOEt, 2.) AcOEt, ether; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
75530-60-8
isopropyl 2-formyl-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-5-carboxylate

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate; acetic anhydride 1.) AcOH, RT, 2.5 h, 2.) AcOH, a) RT, 1.5 h, b) 95 deg C - 100 deg C, 4 h; Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1: hydroxylamine hydrochloride; sodium acetate / water; methanol / 4 h / 20 °C 2: acetic anhydride; potassium carbonate / dimethyl sulfoxide / 5 h / 10 - 50 °C View Scheme |
-
-
39562-25-9
3-oxo-2-(3-nitrophenylmethylene)butanoic acid isopropyl ester

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| With sodium methylate In isopropyl alcohol Heating; |
-
-
542-08-5
isopropyl acetoacetate

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / piperidinium acetate / propan-2-ol / 10 h / 45 - 55 °C 2: sodium methoxide / propan-2-ol / Heating View Scheme |
-
-
99-61-6
3-nitro-benzaldehyde

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / piperidinium acetate / propan-2-ol / 10 h / 45 - 55 °C 2: sodium methoxide / propan-2-ol / Heating View Scheme |
-
-
60705-25-1
4,4-dimethoxy-3-oxo-butyric acid methyl ester

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: piperidine / benzene / 3 h / Heating 2: 51.1 percent / 8.5 h / 95 - 120 °C 3: 95.4 percent / 6 N aq. HCl / acetone / 1 h / Ambient temperature 4: 1.) NH2OH*HCl, NaOAc, 2.) Ac2O / 1.) AcOH, RT, 2.5 h, 2.) AcOH, a) RT, 1.5 h, b) 95 deg C - 100 deg C, 4 h View Scheme |
-
-
14205-46-0
isopropyl 3-aminocrotonate

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 51.1 percent / 8.5 h / 95 - 120 °C 2: 95.4 percent / 6 N aq. HCl / acetone / 1 h / Ambient temperature 3: 1.) NH2OH*HCl, NaOAc, 2.) Ac2O / 1.) AcOH, RT, 2.5 h, 2.) AcOH, a) RT, 1.5 h, b) 95 deg C - 100 deg C, 4 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: piperidine / benzene / 3 h / Heating 2: 51.1 percent / 8.5 h / 95 - 120 °C 3: 95.4 percent / 6 N aq. HCl / acetone / 1 h / Ambient temperature 4: 1.) NH2OH*HCl, NaOAc, 2.) Ac2O / 1.) AcOH, RT, 2.5 h, 2.) AcOH, a) RT, 1.5 h, b) 95 deg C - 100 deg C, 4 h View Scheme |
-
-
67448-15-1
(E,Z)-methyl 4,4-dimethoxy-2-(3-nitrobenzylidene)acetoacetate

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 51.1 percent / 8.5 h / 95 - 120 °C 2: 95.4 percent / 6 N aq. HCl / acetone / 1 h / Ambient temperature 3: 1.) NH2OH*HCl, NaOAc, 2.) Ac2O / 1.) AcOH, RT, 2.5 h, 2.) AcOH, a) RT, 1.5 h, b) 95 deg C - 100 deg C, 4 h View Scheme |
-
-
75530-94-8
isopropyl 2-dimethoxymethyl-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-5-carboxylate

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95.4 percent / 6 N aq. HCl / acetone / 1 h / Ambient temperature 2: 1.) NH2OH*HCl, NaOAc, 2.) Ac2O / 1.) AcOH, RT, 2.5 h, 2.) AcOH, a) RT, 1.5 h, b) 95 deg C - 100 deg C, 4 h View Scheme |
-
-
113201-61-9
2-cyano-1,4-dihydro-3-methoxycarbonyl-6-methyl-4-m-nitrophenylpyridine-5-carboxylic acid

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride | |
| With phosphorus pentachloride |
-
-
497-19-8
sodium carbonate

-
-
113201-61-9
2-cyano-1,4-dihydro-3-methoxycarbonyl-6-methyl-4-m-nitrophenylpyridine-5-carboxylic acid

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In dichloromethane; ethyl acetate; benzene |
-
-
75530-68-6
nilvadipine

-
-
99935-62-3
isopropyl 2-cyano-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)pyridine-5-carboxylate

| Conditions | Yield |
|---|---|
| With nitric acid; acetic acid for 3h; Ambient temperature; | 93.8% |
-
-
75530-68-6
nilvadipine

-
-
145220-80-0
isopropyl 2-carbamoyl-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-1, 4-dihydropyridine-5-carboxylate

| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.166667h; | 87.9% |
-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| With lithium iodide In pyridine for 6h; Heating; |
-
-
811-98-3
d(4)-methanol

-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| With pyridine; 2-bromo-1-methyl-pyridinium iodide; N,N-dimethyl-aniline; lithium iodide 1.) 110 deg C, 8 h, 2.) DMF, RT, 7.5 h; Yield given. Multistep reaction; |
-
-
75530-68-6
nilvadipine

-
-
145220-88-8
methyl 2-cyano-4-(3-nitrophenyl)-5-oxo-5,7-dihydrofuro<3,4-b>pyridine-3-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: 58.9 percent / 30percent aq. H2O2, AcOH / 312 h / 70 °C 3: 91.8 percent / p-TsOH / 1 h / Heating 4: 60.5 percent / p-TsOH / methanol / 15.5 h / Heating View Scheme |
-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: LiI / pyridine / 5 h / Heating View Scheme |
-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 87.9 percent / 80percent aq. H2SO4 / 0.17 h 2: 79.8 percent / MnO2 / CHCl3 / 5 h / Ambient temperature View Scheme |
-
-
75530-68-6
nilvadipine

-
-
145220-89-9
2-cyano-5-isopropoxycarbonyl-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)pyridine-N-oxide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: 58.9 percent / 30percent aq. H2O2, AcOH / 312 h / 70 °C View Scheme |
-
-
75530-68-6
nilvadipine

-
-
145220-90-2
isopropyl 6-acetoxymethyl-2-cyano-3-methoxycarbonyl-4-(3-nitrophenyl)pyridine-5-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: 58.9 percent / 30percent aq. H2O2, AcOH / 312 h / 70 °C 3: 91.8 percent / p-TsOH / 1 h / Heating View Scheme |
-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: 58.9 percent / 30percent aq. H2O2, AcOH / 312 h / 70 °C 3: 91.8 percent / p-TsOH / 1 h / Heating 4: 60.5 percent / p-TsOH / methanol / 15.5 h / Heating 5: 38.2 percent / LiI / pyridine / 2.5 h / 100 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: 58.9 percent / 30percent aq. H2O2, AcOH / 312 h / 70 °C 3: 91.8 percent / p-TsOH / 1 h / Heating 4: 86.4 percent / LiI / pyridine / 3 h / 100 °C 5: 13.7 percent / LiOH*H2O / methanol / 3 h / Heating View Scheme |
-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: 58.9 percent / 30percent aq. H2O2, AcOH / 312 h / 70 °C 3: 91.8 percent / p-TsOH / 1 h / Heating 4: 60.5 percent / p-TsOH / methanol / 15.5 h / Heating 5: 74.6 percent / aq. NaHCO3 / methanol / 11 h / Heating View Scheme |
-
-
75530-68-6
nilvadipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 93.8 percent / aq. HNO3, AcOH / 3 h / Ambient temperature 2: 58.9 percent / 30percent aq. H2O2, AcOH / 312 h / 70 °C 3: 91.8 percent / p-TsOH / 1 h / Heating 4: 86.4 percent / LiI / pyridine / 3 h / 100 °C View Scheme |
Nilvadipine Specification
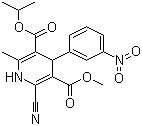
The Nilvadipine, with the CAS registry number 75530-68-6, is also known as 5-Isopropyl 3-methyl 2-cyano-1,4-dihydro-6-methyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate. It belongs to the product categories of Inhibitors; Intermediates & Fine Chemicals; Pharmaceuticals. This chemical's molecular formula is C19H19N3O6 and molecular weight is 385.37. Its IUPAC name is called 3-O-methyl 5-O-propan-2-yl 2-cyano-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate.
Physical properties of Nilvadipine: (1)ACD/LogP: 1.72; (2)ACD/LogD (pH 5.5): 1.72; (3)ACD/LogD (pH 7.4): 1.72; (4)ACD/BCF (pH 5.5): 12.03; (5)ACD/BCF (pH 7.4): 12.03; (6)ACD/KOC (pH 5.5): 206.47; (7)ACD/KOC (pH 7.4): 206.47; (8)#H bond acceptors: 9; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 7; (11)Index of Refraction: 1.584; (12)Molar Refractivity: 97.1 cm3; (13)Molar Volume: 290 cm3; (14)Surface Tension: 57 dyne/cm; (15)Density: 1.32 g/cm3; (16)Flash Point: 272.3 °C; (17)Enthalpy of Vaporization: 80.11 kJ/mol; (18)Boiling Point: 526.7 °C at 760 mmHg; (19)Vapour Pressure: 3.5E-11 mmHg at 25°C.
Preparation: this chemical can be prepared by 3-nitrobenzaldehyde, piperidine, 4,4 - dimethoxy methyl acetoacetate and acetic acid. This reaction will need 2-amino-2-crotonic acid isopropyl.
.png)
.png)
Uses of Nilvadipine: it is a calcium channel blocker (CCB) for the treatment of hypertension and chronic major cerebral artery occlusion. It can be used as an antihypertensive and antianginal. it can be used as calcium antagonists of dihydropyridine. Besides, it also can be used for essential hypertension. This chemical's classification codes are Antagonist [calcium channel]; Antihypertensive agents; Calcium channel blockers; Cardiovascular Agents; Drug / Therapeutic Agent; Membrane Transport Modulators; Reproductive Effect.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC(C)C)\C1=C(\NC(/C#N)=C(/C(=O)OC)C1c2cccc([N+]([O-])=O)c2)C
(2)InChI: InChI=1/C19H19N3O6/c1-10(2)28-19(24)15-11(3)21-14(9-20)17(18(23)27-4)16(15)12-6-5-7-13(8-12)22(25)26/h5-8,10,16,21H,1-4H3
(3)InChIKey: FAIIFDPAEUKBEP-UHFFFAOYAX
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | 3850ug/kg (3.85mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Kiso to Rinsho. Clinical Report. Vol. 21, Pg. 1723, 1987. |
| dog | LD50 | oral | 510mg/kg (510mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Kiso to Rinsho. Clinical Report. Vol. 21, Pg. 1723, 1987. |
| mouse | LD50 | intravenous | 9150ug/kg (9.15mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| mouse | LD50 | oral | 1300mg/kg (1300mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Kiso to Rinsho. Clinical Report. Vol. 21, Pg. 1723, 1987. |
| mouse | LD50 | subcutaneous | > 320mg/kg (320mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| rat | LD50 | intravenous | 9650ug/kg (9.65mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| rat | LD50 | oral | 1560mg/kg (1560mg/kg) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Kiso to Rinsho. Clinical Report. Vol. 21, Pg. 1723, 1987. |
| rat | LD50 | subcutaneous | > 1gm/kg (1000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View