-
Name
L-NORADRENALINE HYDROCHLORIDE
- EINECS
- CAS No. 329-56-6
- Article Data2
- CAS DataBase
- Density 1.397g/cm3
- Solubility
-
Melting Point
~150 °C (dec.)
- Formula C8H11 N O3 . Cl H
- Boiling Point 442.6°Cat760mmHg
- Molecular Weight 205.641
- Flash Point 221.5°C
- Transport Information
- Appearance
- Safety Poison by ingestion, subcutaneous, and intraduodenal routes. When heated to decomposition it emits very toxic fumes of HCl and NOx. See also l-NOREPINEPHRINE.
- Risk Codes 25
-
Molecular Structure
-
Hazard Symbols

- Synonyms 1,2-Benzenediol,4-(2-amino-1-hydroxyethyl)-, hydrochloride, (R)-; 1,2-Benzenediol,4-[(1R)-2-amino-1-hydroxyethyl]-, hydrochloride (9CI); Benzyl alcohol, a-(aminomethyl)-3,4-dihydroxy-,hydrochloride, (-)- (8CI); (-)-Noradrenaline hydrochloride; (-)-Norepinephrinehydrochloride; Arterenol hydrochloride; Aterenol; Noradrenaline hydrochloride;Norepinephrine hydrochloride; R-(-)-Noradrenaline hydrochloride;R-(-)-Norepinephrine monohydrochloride; l-Arterenol hydrochloride;l-Noradrenaline hydrochloride; l-Norepinephrine hydrochloride
- PSA 86.71000
- LogP 1.59220
Synthetic route

| Conditions | Yield |
|---|---|
| In d(4)-methanol; water-d2 Equilibrium constant; |
-
-
1031721-23-9
N-{{7-[bis(carboxymethyl)amino]coumarin-4-yl}methyloxycarbonyl}-L-norepinephrine

-
A
-
876275-37-5
7-[bis(carboxymethyl)amino]-4-(hydroxymethyl)coumarin

-
B
-
124-38-9
carbon dioxide

-
C
-
329-56-6
norepinephrine hydrochloride

| Conditions | Yield |
|---|---|
| With potassium hydroxide; HEPES buffer In acetonitrile pH=7.2; Quantum yield; UV-irradiation; |

-
-
329-56-6
norepinephrine hydrochloride

-
-
79539-35-8
4-amino-3,6-disulfo-1,8-naphthaldehyde anhydride dipotassium salt

| Conditions | Yield |
|---|---|
| In water Heating; pH 5, Li(1+)/H(1+) acetate buffer; |
-
-
52-90-4
L-Cysteine

-
-
329-56-6
norepinephrine hydrochloride

-
A
-
175482-45-8
7-(1-Hydroxy-2-aminoethyl)-3,4-dihydro-5-hydroxy-2H-1,4-benzothiazine-3-carboxylic acid


| Conditions | Yield |
|---|---|
| With phosphate buffer for 0.5h; electro-oxidation reaction; Further byproducts given; |

-
-
52-90-4
L-Cysteine

-
-
329-56-6
norepinephrine hydrochloride

-
A
-
175482-45-8
7-(1-Hydroxy-2-aminoethyl)-3,4-dihydro-5-hydroxy-2H-1,4-benzothiazine-3-carboxylic acid


| Conditions | Yield |
|---|---|
| With phosphate buffer for 0.5h; electro-oxidation reaction; Further byproducts given; |

-
-
52-90-4
L-Cysteine

-
-
329-56-6
norepinephrine hydrochloride

-
A
-
175482-45-8
7-(1-Hydroxy-2-aminoethyl)-3,4-dihydro-5-hydroxy-2H-1,4-benzothiazine-3-carboxylic acid


| Conditions | Yield |
|---|---|
| With phosphate buffer for 0.5h; electro-oxidation reaction; Further byproducts given; |

-
-
329-56-6
norepinephrine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.5h; electrochemical oxidation; |
-
-
52-90-4
L-Cysteine

-
-
329-56-6
norepinephrine hydrochloride



-
C
-
196309-90-7
(R)-7-[(R)-2-((R)-2-Amino-2-carboxy-ethyldisulfanyl)-1-carboxy-ethylamino]-10-((R)-2-amino-2-carboxy-ethylsulfanyl)-9-hydroxy-1,2,3,6-tetrahydro-4,5-dithia-1,8-diaza-phenanthrene-2-carboxylic acid

| Conditions | Yield |
|---|---|
| pH 7.4; |


| Conditions | Yield |
|---|---|
| In d(4)-methanol; water-d2 Equilibrium constant; Thermodynamic data; |
-
-
329-56-6
norepinephrine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 0.1M aq. HCl / 0.5 h / electrochemical oxidation 2: 0.1N HCl View Scheme |
-
-
329-56-6
norepinephrine hydrochloride

-
-
115331-10-7
5-S-Cysteinylepinephrine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 0.1M aq. HCl / 0.5 h / electrochemical oxidation 2: 0.1N HCl View Scheme |

-
-
329-56-6
norepinephrine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform; dimethyl sulfoxide | 110% |
-
-
937021-61-9
C29H32N2O11

-
-
329-56-6
norepinephrine hydrochloride

-
-
1031721-30-8
N-{{7-[bis(tert-butoxycarbonylmethyl)amino]coumarin-4-yl}methyloxycarbonyl}-L-norepinephrine

| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide at 20℃; for 3.5h; | 70 mg |

| Conditions | Yield |
|---|---|
| In water-d2 at 24.84℃; pH=7.1; aq. phosphate buffer; |

-
-
329-56-6
norepinephrine hydrochloride

| Conditions | Yield |
|---|---|
| In methanol |
Norepinephrine hydrochloride Chemical Properties
IUPAC Name: [2-(3,4-Dihydroxyphenyl)-2-hydroxyethyl]azanium chloride
Synonyms: 1,2-Benzenediol, 4-[(1R)-2-amino-1-hydroxyethyl]-, hydrochloride (1:1) ; 4-[(1R)-2-Amino-1-hydroxyethyl]benzene-1,2-diol hydrochloride (1:1) ; Aktamin hydrochloride ; Levophed hydrochloride
CAS NO:329-56-6
Molecular Formula of Norepinephrine hydrochloride (CAS NO.329-56-6) :C8H12ClNO3
Molecular Weight of Norepinephrine hydrochloride (CAS NO.329-56-6) :205.64
Molecular Structure of Norepinephrine hydrochloride (CAS NO.329-56-6) :
EINECS: 222-395-0 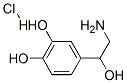
Flash Point: 221.5 °C
Enthalpy of Vaporization: 73.79 kJ/mol
Boiling Point: 442.6 °C at 760 mmHg
Vapour Pressure: 1.3E-08 mmHg at 25°C
Melting point: ~150 °C (dec.)
Storage temp: 2-8°C
Norepinephrine hydrochloride Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 5mg/kg (5mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 99, Pg. 45, 1950. | |
| rat | LD50 | intraduodenal | 26mg/kg (26mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 180, Pg. 155, 1969. | |
| rat | LD50 | oral | 132mg/kg (132mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 180, Pg. 155, 1969. | |
| rat | LD50 | subcutaneous | 29mg/kg (29mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 180, Pg. 155, 1969. |
Norepinephrine hydrochloride Safety Profile
Poison by ingestion, subcutaneous, and intraduodenal routes. When heated to decomposition it emits very toxic fumes of HCl and NOx. See also l-NOREPINEPHRINE.
Hazard Codes  T,
T, C,
C, F
F
Risk Statements 25-34-11
R11:Highly flammable.
R25 :Toxic if swallowed.
R34:Causes burns.
Safety Statements 45-36/37/39-26-16
S16:Keep away from sources of ignition.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR UN 2811 6.1/PG 3
WGK Germany 3
RTECS DN6475000
F 3-8-10-23
HazardClass 6.1(b)
PackingGroup III
Related Products
- Norepinephrine
- Norepinephrine hydrochloride
- Norepinephrine tartrate
- 329-59-9
- 32961-44-7
- 329-63-5
- 3296-50-2
- 329-65-7
- 3296-90-0
- 32971-25-8
- 329722-47-6
- 32972-46-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View