-
Name
Norepinephrine
- EINECS 200-096-6
- CAS No. 51-41-2
- Article Data12
- CAS DataBase
- Density 1.397 g/cm3
- Solubility Soluble in alkali and dilute hydrochloric acid. Slightly soluble in water, ethanol and diethyl ether.
- Melting Point 220-230 °C
- Formula C8H11NO3
- Boiling Point 442.6 °C at 760 mmHg
- Molecular Weight 169.18
- Flash Point 221.5 °C
- Transport Information UN 2811 6.1/PG 2
- Appearance Off-white to tan solid
- Safety 28-36/37-45-36/37/39-26-16
- Risk Codes 26/27/28-34-11
-
Molecular Structure
-
Hazard Symbols
 T+,
T+, C,
C, F
F
- Synonyms 1,2-Benzenediol,4-(2-amino-1-hydroxyethyl)-, (R)-;Arterenol, l- (6CI);Benzyl alcohol, a-(aminomethyl)-3,4-dihydroxy-,(-)- (8CI);(-)-Arterenol;(-)-Noradrenaline;(-)-Norepinephrine;(-)-a-(Aminomethyl)protocatechuylalcohol;(R)-(-)-Norepinephrine;(R)-Noradrenaline;(R)-Norepinephrine;4-((1R)-2-Amino-1-hydroxyethyl)-1,2-benzenediol;Adrenor;Aktamin;Arterenol;Levoarterenol;Levonor;Levonorepinephrine;Levophed;Nor-Epirenan;Norartrinal;Sympathin E;l-1-(3,4-Dihydroxyphenyl)-2-aminoethanol;l-2-Amino-1-(3,4-dihydroxyphenyl)ethanol;l-3,4-Dihydroxyphenylethanolamine;l-Arterenol;
- PSA 86.71000
- LogP 0.79020
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: arterenone With (-)-diisopinocampheylboron chloride In tert-butyl methyl ether at -25 - -20℃; for 3h; Inert atmosphere; Stage #2: With hydrogenchloride In tert-butyl methyl ether; water at 25℃; for 1.5h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; | 70.3% |
-
-
104819-57-0, 104873-30-5, 104873-31-6
R-(+)-2,2'-dinitrobiphenyl-6,6'-dicarbonsaeure-di-N,N'-1-(3,4-dihydroxyphenyl)-1-hydroxy-2-amido-ethan

-
-
51-41-2
norepinephrine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In ethanol for 6h; Ambient temperature; | 51.4% |

| Conditions | Yield |
|---|---|
| With MANDELIC ACID | |
| With (R)-methoxyphenylacetic acid | |
| With L-Tartaric acid |
-
-
56-40-6
glycine

-
-
139-85-5
3,4-dihydroxybenzaldehyde

-
A
-
51-41-2
norepinephrine

-
B
-
149-95-1
D-noradrenaline

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate In dimethyl sulfoxide at 20℃; for 89h; Enzymatic reaction; Title compound not separated from byproducts.; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 61.4 percent / NaHCO3 / benzene; H2O / 30 h 2: NaBH4 / methanol / 24 h 3: 51.4 percent / H2, conc HCl / Pd/C / ethanol / 6 h / Ambient temperature View Scheme |
-
-
104819-55-8, 104873-27-0, 104873-28-1
R-(+)-2,2'-dinitrobiphenyl-6,6'-dicarbonsaeure-di-N,N'-1-(3,4-dihydroxyphenyl)-1-oxo-2-amido-ethan

-
-
51-41-2
norepinephrine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaBH4 / methanol / 24 h 2: 51.4 percent / H2, conc HCl / Pd/C / ethanol / 6 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: tetrahydrobiopterin; oxygen; tyrosine hydroxylase / Enzymatic reaction 2: aromatic L-amino acid decarboxylase / Enzymatic reaction 3: oxygen; dopamine β-hydroxylase; ascorbic acid / Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aromatic L-amino acid decarboxylase / Enzymatic reaction 2: oxygen; dopamine β-hydroxylase; ascorbic acid / Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| With dopamine β-hydroxylase; oxygen; ascorbic acid Enzymatic reaction; |

-
-
51-41-2
norepinephrine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 10h; | 6.8 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (R)-α,α-diphenylprolinol; sodium tetrahydroborate; chloro-trimethyl-silane / tetrahydrofuran / 24 h / 10 - 30 °C / Inert atmosphere 2: N,N-dimethyl-formamide / 18 h / 85 °C 3: hydrogenchloride / water / 10 h / 20 °C View Scheme |

-
-
51-41-2
norepinephrine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N,N-dimethyl-formamide / 18 h / 85 °C 2: hydrogenchloride / water / 10 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With phase-transitioned bovine serum albumin film-coated capillary column In methanol pH=8; Resolution of racemate; |

| Conditions | Yield |
|---|---|
| With water In ethanol at 20℃; for 12h; | 96.7% |
-
-
50-00-0
formaldehyd

-
-
51-41-2
norepinephrine

-
-
41462-32-2
1,2,3,4-tetrahydro-4,6,7-isoquinolinetriol hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 24℃; for 24h; | 92% |
-
-
51-41-2
norepinephrine

-
-
130120-90-0
3-<<4-<(tert-butyldimethylsilyl)oxy>phenyl>acetyl>thiazolidine-2-thione

-
-
130097-10-8
2-[4-(tert-Butyl-dimethyl-silanyloxy)-phenyl]-N-[(R)-2-(3,4-dihydroxy-phenyl)-2-hydroxy-ethyl]-acetamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; | 82% |
-
-
51-41-2
norepinephrine

-
-
74058-64-3
1-(2-Thioxo-thiazolidin-3-yl)-hexadecan-1-one

-
-
74064-89-4, 94475-55-5
N-hexadecanoyl-L-noradrenaline

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; ethanol for 5h; Ambient temperature; | 51% |
-
-
122853-43-4
2-(2-methoxyphenoxy)-ethyl methanesulphonate

-
-
51-41-2
norepinephrine

-
-
76-05-1
trifluoroacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-methoxyphenoxy)-ethyl methanesulphonate; norepinephrine In dimethyl sulfoxide at 70℃; for 6h; Inert atmosphere; Stage #2: trifluoroacetic acid In methanol; water; dimethyl sulfoxide | 46% |


| Conditions | Yield |
|---|---|
| Stage #1: norepinephrine; 3-methoxy-4-(3-phenylpropoxy)phenethyl methanesulfonate In dimethyl sulfoxide at 70℃; for 6h; Inert atmosphere; Stage #2: trifluoroacetic acid In methanol; water; dimethyl sulfoxide Inert atmosphere; | 38% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol at 20℃; for 20h; | 37% |
-
-
51-41-2
norepinephrine

-
-
373359-53-6
N-{4-[4-(4-oxopiperidin-1-yl)phenylsulfamoyl]phenyl}acetamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol under 258.574 - 1034.3 Torr; | 15% |
-
-
51-41-2
norepinephrine

-
-
84702-73-8
5-azidopentan-2-one

-
-
186303-33-3
1-(R)-(3.4-dihydroxyphenyl)-2-(1-methyl-4-azidobutylamino)ethanol oxalate salt

| Conditions | Yield |
|---|---|
| 15% |
-
-
51-41-2
norepinephrine

-
-
108-24-7
acetic anhydride

-
B
-
67727-64-4
3,4-diacetoxybenzaldehyde

-
C
-
15069-79-1
5,6-diacetoxyindole

| Conditions | Yield |
|---|---|
| Stage #1: norepinephrine With sodium periodate; phosphate buffer In water for 0.0833333h; pH=7.4; Stage #2: acetic anhydride With pyridine | A 7% B 1% C 1% D 3% |


| Conditions | Yield |
|---|---|
| With oxygen; tris hydrochloride In water; dimethyl sulfoxide at 25℃; for 15h; pH=8.0; regioselective reaction; | 6% |

| Conditions | Yield |
|---|---|
| With oxygen; tris hydrochloride In water; dimethyl sulfoxide at 25℃; for 15h; pH=8.0; regioselective reaction; | 2% |

| Conditions | Yield |
|---|---|
| With oxygen; tris hydrochloride In water; dimethyl sulfoxide at 25℃; for 15h; pH=8.0; | 2% |

| Conditions | Yield |
|---|---|
| With pyridine |
-
-
51-41-2
norepinephrine

-
-
26630-36-4
(R)-2-acetylamino-1-(3,4-dimethoxy-phenyl)-ethanol

| Conditions | Yield |
|---|---|
| durch Acetylierung und Umsetzung des Reaktionsprodukts mit Diazomethan; |

| Conditions | Yield |
|---|---|
| With methanol; silver(l) oxide |
-
-
51-41-2
norepinephrine

-
-
541-41-3
chloroformic acid ethyl ester

-
-
99161-02-1, 103565-71-5
((R)-3,4,β-trihydroxy-phenethyl)-carbamic acid ethyl ester
-
-
51-41-2
norepinephrine

-
-
108-24-7
acetic anhydride

-
-
28371-32-6
(R)-2-acetylamino-1-(3,4-diacetoxy-phenyl)-ethanol

| Conditions | Yield |
|---|---|
| With ethanol | |
| In methanol |
-
-
51-41-2
norepinephrine

-
-
83086-06-0
6-oxoheptanoic acid p-methylanilide

-
-
90900-18-8
6-[(E)-(R)-2-(3,4-Dihydroxy-phenyl)-2-hydroxy-ethylimino]-heptanoic acid p-tolylamide

| Conditions | Yield |
|---|---|
| With 3 A molecular sieve In N,N-dimethyl-formamide at 75℃; for 24h; |
-
-
51-41-2
norepinephrine

-
-
84417-40-3
6-oxo-N-<4-(trifluoromethyl)phenyl>heptanamide

-
A
-
90900-17-7
6-[(E)-(R)-2-(3,4-Dihydroxy-phenyl)-2-hydroxy-ethylimino]-heptanoic acid (4-trifluoromethyl-phenyl)-amide

| Conditions | Yield |
|---|---|
| With 3 A molecular sieve In N,N-dimethyl-formamide at 75℃; for 24h; Title compound not separated from byproducts; |

-
-
51-41-2
norepinephrine

-
-
130097-16-4
homovanillic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; |
-
-
51-41-2
norepinephrine

-
-
75-07-0
acetaldehyde

-
-
85507-49-9
trans-1,2,3,4-tetrahydro-1-methyl-4,6,7-isoquinolinetriol hydrochloride

-
-
85507-48-8
cis-1,2,3,4-tetrahydro-1-methyl-4,6,7-isoquinolinetriol hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 36h; Product distribution; with formaldehyde, var. pH; | |
| With hydrogenchloride for 36h; Yield given. Yields of byproduct given; |

-
-
51-41-2
norepinephrine

-
-
204763-28-0
4-diethylaminodiazobenzene-4'-isothiocyanate

| Conditions | Yield |
|---|---|
| With acetic acid; triethylamine In acetone Addition; Heating; |

| Conditions | Yield |
|---|---|
| With Krebs solution; monoamine oxidase in lung of rat Kinetics; Further Variations:; Catalysts; Deamination; |
-
-
51-41-2
norepinephrine

-
-
7732-18-5
water

-
-
107-15-3
ethylenediamine

-
-
28857-14-9
1,2,3,4-tetrahydropyrazino[2,3-g]quinoxaline

| Conditions | Yield |
|---|---|
| pH 11; |

Norepinephrine Specification
The l-Norepinephrine with CAS registry number of 51-41-2 is also known as Noradrenalina. The IUPAC name is 4-[(1R)-2-Amino-1-hydroxyethyl]benzene-1,2-diol. It belongs to product categories of Chiral. Its EINECS registry number is 200-096-6. In addition, the formula is C8H11NO3 and the molecular weight is 169.18. This chemical is a off-white to tan solid and should be stored in sealed containers with protection of argon and away from light at the temperature of 4 °C.
Physical properties about l-Norepinephrine are: (1)ACD/LogP: -0.88; (2)# of Rule of 5 Violations: 1 ; (3)#H bond acceptors: 4; (4)#H bond donors: 5; (5)#Freely Rotating Bonds: 6; (6)Index of Refraction: 1.659; (7)Molar Refractivity: 44.63 cm3; (8)Molar Volume: 121 cm3; (9)Surface Tension: 76.7 dyne/cm; (10)Density: 1.397 g/cm3; (11)Flash Point: 221.5 °C; (12)Enthalpy of Vaporization: 73.79 kJ/mol; (13)Boiling Point: 442.6 °C at 760 mmHg; (14)Vapour Pressure: 1.3E-08 mmHg at 25 °C.
Preparation of l-Norepinephrine. The reaction has two steps. Firstly, collar hydroquinone is mixed with chloroacetyl chloride to generate 3,4-dihydroxy-2-chloro acetophenone. Secondly, the reactant reacts with Ammonia or methenamine to obtain the product.
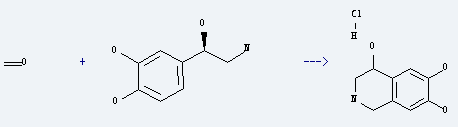
Uses of l-Norepinephrine: it is used to produce 1,2,3,4-tetrahydro-4,6,7-isoquinolinetriol hydrochloride by reaction with formaldehyde. The reaction occurs with reagent 1M HCl at 24 °C for 24 hours. The yield is about 92%. What's more, it is used to as vasoactive drug of anti-shock.
When you are using this chemical, please be cautious about it. As a chemical, it is very toxic by inhalation, in contact with skin and if swallowed. What's more, it is highly flammable that may cause burns. During using it, wear suitable protective clothing, gloves and eye/face protection. Avoid contact with skin and eyes. Keep away from sources of ignition. After contact with skin, wash immediately. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC(=C(C=C1C(CN)O)O)O
2. Isomeric SMILES: C1=CC(=C(C=C1[C@H](CN)O)O)O
3. InChI: InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1
4. InChIKey: SFLSHLFXELFNJZ-QMMMGPOBSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 6mg/kg (6mg/kg) | Drugs in Japan Vol. 6, Pg. 567, 1982. | |
| mouse | LD50 | intravenous | 550ug/kg (0.55mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 226, Pg. 493, 1955. | |
| mouse | LD50 | oral | 20mg/kg (20mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 49, 1972. | |
| mouse | LD50 | subcutaneous | 5mg/kg (5mg/kg) | Naturwissenschaften. Vol. 56, Pg. 615, 1969. | |
| rabbit | LDLo | intravenous | 250ug/kg (0.25mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 41, Pg. 365, 1931. | |
| rat | LD50 | intravenous | 100ug/kg (0.1mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Journal of Pharmacology and Experimental Therapeutics. Vol. 95, Pg. 502, 1949. |
Related Products
- Norepinephrine
- Norepinephrine hydrochloride
- Norepinephrine tartrate
- 5141-20-8
- 51415-00-0
- 51417-51-7
- 51419-40-0
- 51419-51-3
- 51419-59-1
- 514209-42-8
- 51421-22-8
- 51421-98-8
- 5142-22-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View