-
Name
Oxalic acid
- EINECS 205-634-3
- CAS No. 144-62-7
- Article Data1764
- CAS DataBase
- Density 1.772 g/cm3
- Solubility Water solubility: 90 g/L (20 °C)
- Melting Point 189-191 °C
- Formula C2H2O4
- Boiling Point 365.099 °C at 760 mmHg
- Molecular Weight 90.0355
- Flash Point 188.79 °C
- Transport Information UN 3261 8/PG 3
- Appearance Odorless white solid
- Safety 24/25-23-36/37/39-27-26
- Risk Codes 21/22-63-34
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Ethanedioic acid ,Oxalic Acid 99.6%;industry grade Oxalic acid;Oxalic acid, Ethanedioic acid, Dicarboxylic acid;Anhydrous oxalic acid;Oxaluria;Acido ossalico;Aquisal;Oxagel;Ethanedioic acid (9CI);EPA Pesticide Chemical Code 009601;Oxiric acid;Acide oxalique;Aktisal;Kyselina stavelova;Oxalic acid anhy.;Oxalic acid anhydrous;Oxalic Acid Dihydrate;
- PSA 74.60000
- LogP -0.84440
Synthetic route

| Conditions | Yield |
|---|---|
| With oxygen; ethylenediamine; Flavin mononucleotide In water at 15℃; under 4350.3 Torr; for 77h; glycolate oxidase, Aspergillus niger catalase, pH 8-9; | A 0.2% B 99.8% |

| Conditions | Yield |
|---|---|
| With ion exchange resin D001 In water at 75℃; for 1.41667h; Autoclave; Sealed tube; Large scale; | 99.8% |
| With potassium hydroxide In methanol at 35℃; for 0.0833333h; | 96% |
| With sodium hydroxide In N,N-dimethyl-formamide for 0.25h; Ambient temperature; Yield given; |

| Conditions | Yield |
|---|---|
| With ion-exchange resin Indion-130 In water at 75℃; for 1.5h; Reagent/catalyst; Autoclave; Sealed tube; Large scale; | 99.6% |
| With sulfuric acid In methanol; water for 2h; Reflux; |

| Conditions | Yield |
|---|---|
| With diazoacetic acid ethyl ester; oxygen; Flavin mononucleotide In water at 5℃; under 6205.8 Torr; for 1h; Pichia Pastoris transformant MSO10 (423 IU of glycolate oxidase and 869000 IU of catalase), pH 9.25; | A 1.3% B 98.7% |
| With oxygen; 3,3-dimethyldioxirane In acetone at 32℃; Kinetics; Further Variations:; Temperatures; Oxidation; |

| Conditions | Yield |
|---|---|
| With oxygen In water at 249.9℃; under 37503 Torr; for 2h; Mechanism; Product distribution; various conc. NaOH solution,; | A 96.7% B n/a |

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
B
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| Stage #1: [Cu2(m-xpt)2(μ-C2O4)](PF6)2 With nitric acid In methanol for 3h; Stage #2: N,N-dimethyl-formamide In water | A 96% B n/a |


-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
B
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| Stage #1: [Cu2(m-xpt)2(μ-C2O4)](PF6)2 With hydrogenchloride In methanol for 3h; Stage #2: N,N-dimethyl-formamide In water | A 94% B n/a |

-
-
2018-45-3
4-(β-hydroxyethyl)-4-methyl-1,3-dioxane

-
A
-
597-44-4
2-hydroxy-2-methylbutane-1,4-dioic acid

-
B
-
503-49-1
meglutol

-
C
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| With nitric acid 1. adding, 7 h; 50-60 deg C, 1 h; | A 5% B 90% C 4% |


| Conditions | Yield |
|---|---|
| With nitric acid In water at 20℃; for 240h; | 85% |
| With oxygen In acetonitrile at 20℃; for 24h; Catalytic behavior; | 55% |
| With alkaline permanganate at 50℃; |

| Conditions | Yield |
|---|---|
| With ozone | A 10% B 55% C 80% |


| Conditions | Yield |
|---|---|
| With tetramethyl ammoniumhydroxide; tetramethlyammonium chloride In water for 3.25h; pH=9, electrolysis -0.9 V , 1.2 mA, graphite electrode; | 78% |
| With N,N'-bis(2-hydroxy-1-naphthaldehyde)1,3-phenylenediimine In acetonitrile Electrolysis; UV-irradiation; Inert atmosphere; | |
| With samarium; chloro-trimethyl-silane; tetra-(n-butyl)ammonium iodide In acetonitrile at 20℃; under 760.051 Torr; for 2h; Electrochemical reaction; Cooling with ice; |

| Conditions | Yield |
|---|---|
| With oxygen In water at 249.9℃; under 37503 Torr; for 2h; Mechanism; Product distribution; various conc. NaOH solution,; | A 75.4% B n/a |

| Conditions | Yield |
|---|---|
| With water; iodine; sodium hydroxide at 20℃; pH=7; | 75% |
| With Eosin Y Photolysis; | |
| With isochlorophyllyne Photolysis; |

| Conditions | Yield |
|---|---|
| With oxygen In water at 5℃; under 6205.8 Torr; for 23h; Product distribution; Escherichia coli transformant WT-GAO, pH 9.2; other metabolically inactive microbial transformant; | A 5.6% B 1.1% C 74.4% |


| Conditions | Yield |
|---|---|
| With nitric acid; dinitrogen tetraoxide | A 12% B 70% |
-
-
13360-52-6
Cellobiose

-
A
-
79-14-1
glycolic Acid

-
B
-
526-95-4
gluconic acid

-
C
-
110-15-6
succinic acid

-
D
-
144-62-7
oxalic acid

-
E
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With carbon nanotube supported gold nanoparticles (0.5 wt%); water; oxygen at 145℃; under 7500.75 Torr; for 3h; | A n/a B 70% C n/a D n/a E n/a |

-
-
13360-52-6
Cellobiose

-
A
-
79-14-1
glycolic Acid

-
B
-
50-99-7
D-glucose

-
C
-
526-95-4
gluconic acid

-
D
-
110-15-6
succinic acid

-
E
-
144-62-7
oxalic acid

-
F
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With carbon nanotube supported gold nanoparticles (0.5 wt%); water; oxygen at 145℃; under 3750.38 Torr; for 3h; | A n/a B n/a C 68% D n/a E n/a F n/a |

-
-
7722-84-1
dihydrogen peroxide

-
-
56-81-5
glycerol

-
A
-
64-18-6
formic acid

-
B
-
124-38-9
carbon dioxide

-
C
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| iron(III) sulfate In water addition of 2.4% H2O2-soln., storage for 24 hours;; | A 24.77% B 67.8% C <1 |

-
-
124-38-9
carbon dioxide

-
-
15487-82-8, 6735-25-7
triphenylsilylkalium

-
A
-
1450-23-3
hexaphenyldisilane

-
B
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane at 0℃; Product distribution; | A 65% B 10% |


| Conditions | Yield |
|---|---|
| With tetramethyl ammoniumhydroxide; tetramethlyammonium chloride In water for 4.25h; pH=9, electrolysis -1.88 V , 3.0 mA, graphite electrode; | A 65% B 6% C 28% |
| With tetraethylammonium perchlorate In water electrodeduction, cathode: Pb vs. Ag/AgCl; Yield given; |


| Conditions | Yield |
|---|---|
| With oxygen In water at 249.9℃; under 37503 Torr; for 2h; Mechanism; Product distribution; various conc. NaOH solution,; | A 61.6% B 1.5% C n/a |


| Conditions | Yield |
|---|---|
| With oxygen; platinum on activated charcoal In water at 60℃; under 150.01 Torr; for 6h; Product distribution; effect of pH (5 - 11); effect of carbon carrier type; effect of catalyst carrier modification; effect of Pt dispersion; selectivity; other time; | A n/a B n/a C 60% D n/a |
| With oxygen; platinum on activated charcoal In water at 60℃; under 150.01 Torr; for 6h; pH 9.0; Title compound not separated from byproducts; | A n/a B n/a C 60% D n/a |


| Conditions | Yield |
|---|---|
| With iron disulphate; dihydrogen peroxide at 2℃; | A 16% B 12% C 58% |


| Conditions | Yield |
|---|---|
| With oxygen In water at 249.9℃; under 37503 Torr; for 2h; Mechanism; Product distribution; various conc. NaOH solution,; | A 57.9% B 1.1% C n/a |


| Conditions | Yield |
|---|---|
| With oxygen In water at 249.9℃; under 37503 Torr; for 2h; Mechanism; Product distribution; various conc. NaOH solution,; | A 57.8% B 12.3% C n/a |


| Conditions | Yield |
|---|---|
| With boron trifluoride at 65℃; for 0.333333h; | 100% |
| With sulfuric acid for 1h; Reflux; | 95% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 70℃; for 15h; | 95% |

| Conditions | Yield |
|---|---|
| at 150℃; for 8h; Product distribution; Further Variations:; Temperatures; solid-state reaction; | 100% |
| With water for 0.05h; microwave irradiation; | 99% |
| With hydrogenchloride In water at 100℃; for 0.333333h; | 98% |
-
-
124-46-9, 20734-13-8, 100224-74-6, 593-85-1
guanidine hydrogen carbonate

-
-
144-62-7
oxalic acid

- tetraethylammonium hydroxide
-
77-98-5
tetraethylammonium hydroxide

| Conditions | Yield |
|---|---|
| In water for 0.0833333h; | 100% |


-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |

-
-
144-62-7
oxalic acid

-
-
346591-05-7
(2S)-(+)-3-[(2S,4S)-4-(5-Chlorobenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-1-(1H-indol-4-yl)oxy-2-propanol oxalate

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |
-
-
144-62-7
oxalic acid

-
-
346589-02-4
(2S)-3-[(2S,4R)-4-(5-chlorobenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-1-(1H-indol-4-yl)oxy-2-propanol

-
-
346589-03-5
(2S)-3-[(2S,4R)-4-(5-Chlorobenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-1-(1H-indol-4-yl)oxy-2-propanol oxalate

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |
-
-
144-62-7
oxalic acid

-
-
346591-06-8
(2S)-(+)-3-[(2R,4S)-4-(5-chlorobenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-1-(1H-indol-4-yl)oxy-2-propanol

-
-
346591-07-9
(2S)-(+)-3-[(2R,4S)-4-(5-Chlorobenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-1-(1H-indol-4-yl)oxy-2-propanol oxalate

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |
-
-
144-62-7
oxalic acid

-
-
346589-06-8
(2S)-1-(1H-indol-4-yl)oxy-3-[(2S,4R)-4-(4-methylbenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-2-propanol

-
-
346589-07-9
(2S)-1-(1H-Indol-4-yl)oxy-3-[(2S,4R)-4-(4-methylbenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-2-propanol oxalate

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |
-
-
144-62-7
oxalic acid

-
-
346591-10-4
(2S)-(-)-1-(1H-indol-4-yl)oxy-3-[(2R,4S)-4-(4-methylbenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-2-propanol

-
-
346591-11-5
(2S)-(-)-1-(1H-Indol-4-yl)oxy-3-[(2R,4S)-4-(4-methylbenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-2-propanol oxalate

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |

-
-
144-62-7
oxalic acid

-
-
346591-13-7
(2S)-3-[(2S,4S)-4-(4,5-Dimethoxybenzo[b]thiophen-2-yl)-2-methylpiperidinyl]-1-(1H-indol-4-yl)oxy-2-propanol oxalate

| Conditions | Yield |
|---|---|
| In ethyl acetate | 100% |

| Conditions | Yield |
|---|---|
| In not given byproducts: CO2; prolonged heating;; | 100% |
| In not given |

| Conditions | Yield |
|---|---|
| In water heating of Rb-alum and oxalic acid in water at boiling temp.; cooling down;; crystn.;; | 100% |
| In water heating of Rb-alum and oxalic acid in water at boiling temp.; cooling down;; crystn.;; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: C19H22Cl2N2*2ClH With 2-Iodophenol; potassium carbonate; potassium iodide In water; toluene at 95℃; for 24h; Stage #2: oxalic acid In ethyl acetate; toluene | 100% |
-
-
1198092-67-9
(E)-N-(2,2-dimethyl-5-phenylpent-4-yn-1-ylidene)benzylamine

-
-
144-62-7
oxalic acid

-
-
116673-95-1
5-benzyl-3,3-dimethyl-3,4-dihydro-2H-pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: (E)-N-(2,2-dimethyl-5-phenylpent-4-yn-1-ylidene)benzylamine With potassium 3,7-dimethyloctan-3-olate In tetrahydrofuran at 0℃; for 1h; Stage #2: oxalic acid With water In toluene for 0.5h; Reflux; Stage #3: With silver tetrafluoroborate In dichloromethane at 40℃; for 6h; | 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

-
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| In water-d2 | 100% |
| In water-d2 | 100% |
Oxalic acid Chemical Properties
Molecular Formula: H2C2O4
Molecular Weight: 90.03 g/mol
EINECS: 205-634-3
Density: 1.9 g/mL
Flashing point: 101-157 °C
Melting point: 189-191 °C
Water solubility: 90 g/L (20 °C)
Appearance: Odorless white solid
Structure of Oxalic acid (CAS NO.144-62-7):
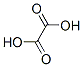
IUPAC Name: Oxalic acid
Product Category of Oxalic acid (CAS NO.144-62-7): Intermediates;Water Ttreatment Chemicals;Alphabetic;Carbonyl Compounds;Carboxylic Acids;Complexometric Solutions
Oxalic acid Uses
Oxalic acid (CAS NO.144-62-7) is used as Bar Keeper's Friend, Zud, some bleaches, and rustproofing treatments, and it is also used as an cadditive to automotive wheel leaners. It is used in platinotype, the early photographic Platinum/Palladium printing process. Oxalic acid is used as a rust remover in such applications as automotive shops and for the restoration of antiques, and as a recommended surface pretreatment for stainless steels (surface etch) before application of solid metal or polymer self-lubricating coatings. It is used for polishing stones and marble, and used in the acid treatment for destroying warts.
Oxalic acid Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MLD | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,311. | ||
| 2. | eye-rbt 250 μg/24H SEV | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,311. | ||
| 3. | eye-rbt 100 mg/4S rns SEV | FCTOD7 Food and Chemical Toxicology. 20 (1982),573. | ||
| 4. | ipr-mus LD50:270 mg/kg | TXCYAC Toxicology. 62 (1990),203. | ||
| 5. | orl-rat LD50:7500 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 42 (1977),417. | ||
| 6. | scu-cat LDLo:112 mg/kg | HBAMAK Abdernaldens Handbuch der Biologischen Arbeitsmethoden. 4 (1935),1377. | ||
| 7. | scu-frg LDLo:757 mg/kg | HBAMAK Abdernaldens Handbuch der Biologischen Arbeitsmethoden. 4 (1935),1377. |
Oxalic acid Consensus Reports
Reported in EPA TSCA Inventory.
Oxalic acid Safety Profile
Poison by subcutaneous route. Moderately toxic by ingestion. A skin and severe eye irritant. Acute oxalic poisoning results from ingestion of a solution of the acid. There is marked corrosion of the mouth, esophagus, and stomach, with symptoms of vomiting, burning abdominal pain, collapse, and sometimes convulsions. Death may follow quickly. The systemic effects are attributed to the removal by the oxalic acid of the calcium in the blood. The renal tubules become obstructed by the insoluble calcium oxalate, and there is profound kidney disturbance. The chief effects of inhalation of the dusts or vapor are severe irritation of the eyes and upper respiratory tract, gastrointestinal disturbances, albuminuria, gradual loss of weight, increasing weakness and nervous system complaints, ulceration of the mucous membranes of the nose and throat, epistaxis, headache, irritation, and nervousness. Oxalic acid has a caustic action on the skin and may cause dermatitis; a case of early gangrene of the fingers resembling that caused by phenol has been described. More severe cases may show albuminuria, chronic cough, vomiting, pain in the back, and gradual emaciation and weakness. The skin lesions are characterized by cracking and fissuring of the skin and the development of slow-healing ulcers. The skin may be bluish in color, and the nails brittle and yellow. Violent reaction with furfuryl alcohol, Ag, NaClO3, NaOCl. When heated to decomposition it emits acrid smoke and irritating fumes. See also OXALATES.
Hazard Codes:  Xn
Xn
Risk Statements: 21/22-63-34
R21/22:Harmful in contact with skin and if swallowed.
R34:Causes burns.
R63:Possible risk of harm to the unborn child.
Safety Statements: 24/25-23-36/37/39-27-26
S23:Do not breathe vapour.
S24/25:Avoid contact with skin and eyes.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S27:Take off immediately all contaminated clothing.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
Oxalic acid Specification
Oxalic acid , its cas register number is 144-62-7. It also can be called Ethanedionic acid ; and Oxalic acid anhydrous . It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately. Notes to physician: Treat supportively and symptomatically.
In addition, Oxalic acid (CAS NO.144-62-7) absorbs moisture or water from the air. It is not compatible with Metals, strong oxidizing agents, strong bases, acid chlorides, sodium hypochlorite, steel, mercury, silver, sodium chloride, chlorites, and you must not take it with incompatible materials. And also prevent it to broken down into hazardous decomposition products: formic acid, carbon monoxide, carbon dioxide.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View