-
Name
Oxindole
- EINECS 200-429-5
- CAS No. 59-48-3
- Article Data216
- CAS DataBase
- Density 1.198 g/cm3
- Solubility insoluble in water
- Melting Point 123-128 °C(lit.)
- Formula C8H7NO
- Boiling Point 312.7 °C at 760 mmHg
- Molecular Weight 133.15
- Flash Point 177.8 °C
- Transport Information UN 2811
- Appearance beige to yellowish-orange or pinkish-brown powder
- Safety 22-24/25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2-Indolinone(7CI,8CI);Oxindole (6CI);1,3-Dihydro-2H-indol-2-one;1,3-Dihydroindol-2-one;2-Indolone;2-Oxindole;2-Oxo-2,3-dihydroindole;2-Oxoindole;2-Oxoindoline;Indol-2(3H)-one;Indoline-2-one;NSC 274863;Oxindol;
- PSA 29.10000
- LogP 1.31920
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: indole-2,3-dione With hydrazine In methanol Heating; Stage #2: With sodium ethanolate In ethanol Wolff-Kishner reduction; Heating; Further stages.; | 98% |
| With hydrogen In ethanol at 70℃; under 1875.19 Torr; for 0.333333h; | 96.5% |
| With titanium tetrachloride; zinc In tetrahydrofuran at 20℃; for 0.0833333h; Temperature; Reagent/catalyst; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With iron; acetic acid at 100℃; | 95% |
| With palladium on activated charcoal; hydrogen; acetic acid at 20℃; for 4h; | 88% |
| With 5%-palladium/activated carbon; hydrogen In ethanol; water under 7500.75 Torr; for 24h; Autoclave; | 88% |
-
-
30095-98-8
methyl (2-nitrophenyl)acetate

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| Stage #1: methyl (2-nitrophenyl)acetate With hydrogen In ethyl acetate at 20℃; under 7600.51 Torr; for 24h; Autoclave; Stage #2: In ethyl acetate for 6h; Reflux; Molecular sieve; | 99% |
| With iron; ammonium chloride; acetic acid at 100℃; for 24h; | 87.8% |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / Inert atmosphere 2: potassium carbonate / N,N-dimethyl-formamide / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / methanol / 25 °C / Inert atmosphere 2: potassium carbonate / N,N-dimethyl-formamide / 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 20 °C / 2280.15 Torr / Sealed tube 2: ethyl acetate / 6 h / Reflux; Molecular sieve View Scheme |

| Conditions | Yield |
|---|---|
| With chloro(meso-tetrakis(2,6-dichlorophenyl)porphyrinato)manganese(III); dihydrogen peroxide In acetonitrile at 20℃; for 1h; | A 44% B 8% |
| With oxygen; titanium(IV) oxide In water for 4h; Irradiation; | |
| With D-glucose; GDH; cytochrome P450 monooxygenase CYP116B4 from Labrenzia aggregata; nicotinamide adenine dinucleotide phosphate In aq. buffer at 30℃; for 5h; pH=8.5; Enzymatic reaction; | |
| With nicotinamide adenine dinucleotide phosphate; cytochrome P450 monooxygenase from Labrenzia aggregata In aq. buffer at 30℃; for 5h; pH=8.5; Enzymatic reaction; enantioselective reaction; |
-
-
1429481-71-9
1-(toluene-4-sulfonyl)-1,3-dihydro-indol-2-one

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With naphthalene radical anion sodium salt In 1,2-dimethoxyethane at -78 - 18℃; Reagent/catalyst; | 90% |

| Conditions | Yield |
|---|---|
| With chloro[2-nitro-5,10,15,20-tetrakis(2,6-diclorophenyl)porphyrinate]manganese(III); chloro[5,10,15,20-tetrakis(4-dimethy-lamino-2,3,5,6-tetrafluorophenyl)porphyrinate]manganese(III) In acetonitrile at 20℃; for 0.5h; | A 35% B 7% |
| With ammonium persulfate; phosphoric acid In water pH=1.4; Oxidation; | A 26% B 32% |
-
-
31912-02-4
ethyl 2-nitrophenylacetate

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With acetic acid; zinc In ethanol at 70℃; for 4h; Inert atmosphere; | 93% |
| With acetic acid; zinc In ethanol at 70℃; for 4h; | 66% |

| Conditions | Yield |
|---|---|
| With chloro(meso-tetrakis(2,6-dichlorophenyl)porphyrinato)manganese(III); dihydrogen peroxide In acetonitrile at 20℃; for 0.5h; | A 64% B 7% C 6% |

-
-
120-72-9
indole

-
A
-
59-48-3
2-oxoindole

-
B
-
91-56-5
indole-2,3-dione

-
C
-
17646-95-6
2,2-di(3-indolyl)-3-indolone

| Conditions | Yield |
|---|---|
| With chloro(meso-tetrakis(2,6-dichlorophenyl)porphyrinato)manganese(III); chloro[2-nitro-5,10,15,20-tetrakis(2,6-diclorophenyl)porphyrinate]manganese(III) In acetonitrile at 20℃; for 0.5h; | A 50% B 6% C 6% |

-
-
171359-95-8
N'-(2-aminobenzylidene)-4-methylbenzenesulfonohydrazide

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane at 20 - 110℃; for 1h; Solvent; Reagent/catalyst; | 83% |
-
-
3740-52-1
2-(2-nitrophenyl)acetic acid

-
A
-
59-48-3
2-oxoindole

-
B
-
18108-55-9
1,3-dihydro-1-hydroxyindole-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-nitrophenyl)acetic acid With 1,10-Phenanthroline; platinum on carbon; hydrogen In ethanol at 20℃; under 7500.75 Torr; Flow reactor; Green chemistry; Stage #2: With acetic acid In ethanol at 20℃; Catalytic behavior; Reagent/catalyst; Solvent; Pressure; Green chemistry; | A n/a B 83% |
| With sulfuric acid; zinc at 30℃; | |
| With acetic acid; platinum Hydrogenation; |

| Conditions | Yield |
|---|---|
| With potassium carbonate; [pentamethylcyclopentadienylRhCl2]2 In acetone at 100℃; for 8h; | 74% |
| Multi-step reaction with 3 steps 1: 1.) aq. HCl, NaNO2, 2.) NaN3 2: 50 percent / Pyridine, CF3COOH, Dicyclohexylcarbodiimide / dimethylsulfoxide; benzene / 0.5 h / 50 °C 3: 85 percent / decahydronaphthalene / 2 h / 190 - 200 °C View Scheme |

| Conditions | Yield |
|---|---|
| With sodium In ethanol Reflux; | 69% |
| 1,4-diaza-bicyclo[2.2.2]octane In 2-Ethylhexyl alcohol; water at 130 - 145℃; for 3.5 - 5h; Heating / reflux; | 66.5% |
| 1,4-diaza-bicyclo[2.2.2]octane In 2-Ethylhexyl alcohol; hexan-1-ol at 130℃; for 2.5h; | 66.5% |
| at 255℃; under 90 Torr; | |
| With potassium tert-butylate In ethanol for 2h; Reflux; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With silver(I) acetate; palladium diacetate; trifluoroacetic acid at 120℃; for 24h; Sealed tube; Inert atmosphere; Schlenk technique; | 84% |
-
-
628-21-7
1,4-Diiodobutane

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| Stage #1: n-butyllithium; N,N,N,N,-tetramethylethylenediamine With diphosphorus pentasulfide In tetrahydrofuran; hexane at -25℃; for 0.5h; Nitrogen atmosphere; Stage #2: 1,4-Diiodobutane In tetrahydrofuran; hexane at -25 - 20℃; for 14h; | 50% |

-
-
65816-14-0
1-methoxyindolin-2-one

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 760.051 Torr; for 1h; | 95% |
| With chloro-trimethyl-silane; sodium iodide In acetonitrile at 80℃; for 8h; | 13% |
-
-
35613-44-6
2-aminophenylacetic acid methyl ester

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With 1,2,2,3,4,4-hexamethylphosphetane 1-oxide; diphenylsilane; phenylboronic acid In m-xylene at 120℃; for 4h; Inert atmosphere; Schlenk technique; Sealed tube; | 92% |
| for 0.333333h; Heating; under reduced pressure; | |
| With water; trimethylamine at 30℃; Rate constant; dependency of rate constants on the nature of general base catalyst; |

| Conditions | Yield |
|---|---|
| With rhodium(II) pivalate In water; chlorobenzene at 140℃; for 5h; Reagent/catalyst; Inert atmosphere; | 78% |
| dirhodium tetraacetate In acetonitrile Rearrangement; Wolff; Heating; | 40% |
| With water; acetic acid at 0℃; Irradiation.Mit der Licht einer Bogenlampe; |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran for 3h; Ambient temperature; Irradiation; | 74% |
| With lithium diisopropyl amide 1.) THF, hexane, -78 deg C, 2.) 25 deg C, irradiation, 3 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With 1-fluoro-1,2-phenyliodohydrin-3-(1H)-one In 1,4-dioxane; water at 140℃; for 5h; Reagent/catalyst; | 81% |
| With silica-gel-supported sulfuric acid In dichloromethane at 25℃; for 10h; Reagent/catalyst; Solvent; regioselective reaction; | 78% |
| With dihydrogen peroxide; iron(II) bromide In water at 20℃; Catalytic behavior; Reagent/catalyst; Fenton Reaction; Green chemistry; | 66% |
-
-
120-72-9
indole

-
A
-
59-48-3
2-oxoindole

-
C
-
91-56-5
indole-2,3-dione

-
D
-
17646-95-6
2,2-di(3-indolyl)-3-indolone

-
E
-
61-71-2
dioxindol

| Conditions | Yield |
|---|---|
| With chloro(meso-tetrakis(2,6-dichlorophenyl)porphyrinato)manganese(III); dihydrogen peroxide In acetonitrile at 20℃; for 6h; Overall yield = 99 %; |

-
-
937606-68-3
1-(2-chloroacetyl)indolin-2-one

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With morpholine In ethanol at 20℃; Reagent/catalyst; |
-
-
10268-06-1
2-(2-chlorophenyl)acetamide

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With copper(I) oxide; potassium phosphate; N1,N2-bis(thiophen-2-ylmethyl)oxalamide In tert-butyl alcohol at 130℃; for 24h; | 75% |
-
-
382150-70-1
1,3-di-tert-butyl 2-(2-aminophenyl)propanedioate

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| With acetic acid In methanol at 60℃; for 48h; Temperature; |
-
-
120-72-9
indole

-
A
-
59-48-3
2-oxoindole

-
B
-
479-41-4
indirubin

-
D
-
91-56-5
indole-2,3-dione

-
E
-
17646-95-6
2,2-di(3-indolyl)-3-indolone

-
F
-
61-71-2
dioxindol

| Conditions | Yield |
|---|---|
| With chloro(meso-tetrakis(2,6-dichlorophenyl)porphyrinato)manganese(III); dihydrogen peroxide In acetonitrile at 20℃; for 3h; Overall yield = 91 %; |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper In neat (no solvent) at 100℃; for 16h; Sealed tube; | 80% |
| With ammonia; water; copper diacetate; copper at 165℃; Unter Druck; |
-
-
30095-98-8
methyl (2-nitrophenyl)acetate

-
A
-
59-48-3
2-oxoindole

-
B
-
35613-44-6
2-aminophenylacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate at 0℃; under 735.5 Torr; for 4h; Title compound not separated from byproducts; | |
| With hydrogen In ethyl acetate at 20℃; under 7600.51 Torr; for 24h; Autoclave; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid / benzene / Heating 2: hydrogen / PtO2 / methanol / 2 h / 2585.7 Torr 3: H2O, trimethylamine / 30 °C / dependency of rate constants on the nature of general base catalyst View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogen / PtO2 / methanol / 2 h / 2585.7 Torr 2: H2O, trimethylamine / 30 °C / dependency of rate constants on the nature of general base catalyst View Scheme |

-
-
59-48-3
2-oxoindole

| Conditions | Yield |
|---|---|
| Stage #1: o'-(2,2-difluorovinyl)-p-toluenesulfonanilide With trimethylsilyl trifluoromethanesulfonate; palladium dichloride In 1,1,1,3,3,3-hexafluoropropan-2-ol at 60℃; for 2h; Stage #2: With water; sodium hydrogencarbonate In 1,1,1,3,3,3-hexafluoropropan-2-ol | 90% |

| Conditions | Yield |
|---|---|
| With UiO-66 metal organic framework nanoparticles In neat (no solvent) at 80℃; for 2h; Friedel-Crafts Alkylation; Sealed tube; Darkness; | 100% |
| With pyrrolidine In ethanol for 2h; Reflux; | 94% |
| With [1-(3-sulfonic acid)]propyl-3-methylimidazolium hydrogen sulfate at 80℃; for 1h; | 93% |
-
-
59-48-3
2-oxoindole

-
-
1195-13-7, 56921-07-4, 90154-87-3, 131348-77-1, 131348-78-2, 143679-79-2
cis-7-azabicyclo<4.3.0>nonan-8-one

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; hydrogen In acetic acid at 70℃; under 760.051 Torr; for 48h; | 100% |
| With C33H49ClNRh; hydrogen In 2,2,2-trifluoroethanol at 20℃; under 30003 Torr; for 24h; Autoclave; Molecular sieve; | 99% |
| With C31H47ClNRh; hydrogen In toluene at 25℃; under 37503.8 Torr; for 24h; Catalytic behavior; Solvent; Pressure; Concentration; Temperature; Molecular sieve; Autoclave; Schlenk technique; diastereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetic acid Heating; | 100% |
| With zirconium(IV) chloride In ethanol Reagent/catalyst; Solvent; Temperature; Reflux; | 91% |
| With hydrogenchloride In water; acetic acid for 3h; Reflux; | 88% |
-
-
59-48-3
2-oxoindole

-
-
91107-10-7
4,6-dimethoxy-2,3-diphenyl-1H-indole

-
-
91107-13-0
7-(indol-2'-yl)-4,6-dimethoxy-2,3-diphenylindole

| Conditions | Yield |
|---|---|
| With trifluoromethylsulfonic anhydride In chloroform for 0.5h; Ambient temperature; | 100% |
| With trichlorophosphate In chloroform Heating; | 56% |
| With trichlorophosphate |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(I) thiophene-2-carboxylate; (1S,2S)-N,N'-dimethyl-1,2-diaminocyclohexane In toluene at 85℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| piperidine In ethanol for 14h; Heating / reflux; | 100% |
-
-
59-48-3
2-oxoindole

-
-
280748-43-8
5-formyl-4-methyl-1H-pyrrole-2-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol at 90 - 100℃; | 100% |
| 268 mg (100%) | |
| 268 mg (100%) | |
| 268 mg (100%) |

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; 4-bromo-benzaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |
| Stage #1: 2-oxoindole; 4-bromo-benzaldehyde With piperidine In ethanol for 12h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 20℃; for 1h; | |
| Stage #1: 2-oxoindole; 4-bromo-benzaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; benzaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |
| Stage #1: 2-oxoindole; benzaldehyde With piperidine In ethanol for 3h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; | 87% |
| Stage #1: 2-oxoindole; benzaldehyde With pyrrolidine In methanol for 3h; Reflux; Stage #2: With sodium tetrahydroborate In methanol | 82% |
-
-
59-48-3
2-oxoindole

-
-
1168720-53-3
3-iodo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indazole-6-carbaldehyde

-
-
1247000-64-1
(E)-3-((3-iodo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indazol-6-yl)methylene)indolin-2-one

| Conditions | Yield |
|---|---|
| With piperidine In ethanol at 20 - 70℃; for 2h; | 100% |
| With piperidine In ethanol at 20 - 70℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| Reagent/catalyst; Aldol Condensation; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; benzaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; 4-cyanobenzaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |
| Stage #1: 2-oxoindole; 4-cyanobenzaldehyde With piperidine In ethanol for 3h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 3h; | 48% |
| Stage #1: 2-oxoindole; 4-cyanobenzaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 20℃; for 2h; |
-
-
455-19-6
4-Trifluoromethylbenzaldehyde

-
-
59-48-3
2-oxoindole

-
-
931927-97-8
3-(4-trifluoromethylbenzyl)-1,3-dihydroindol-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 4-Trifluoromethylbenzaldehyde; 2-oxoindole With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |
| Stage #1: 4-Trifluoromethylbenzaldehyde; 2-oxoindole With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; 3,4-dimethoxy-benzaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; β-naphthaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |
-
-
59-48-3
2-oxoindole

-
-
15971-29-6
4-methoxy-1-naphthaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; 4-methoxy-1-naphthaldehyde With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |
-
-
59-48-3
2-oxoindole

-
-
78646-50-1
2-ethenyl-1-benzothiophene

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxoindole; 2-ethenyl-1-benzothiophene With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromo-2-furancarboxaldehyde; 2-oxoindole With pyrrolidine In ethanol for 2h; Reflux; Stage #2: With sodium tetrahydroborate In ethanol at 0 - 20℃; for 2h; | 100% |
-
-
59-48-3
2-oxoindole

-
-
27508-96-9
5-methoxy-7-methoxyindole-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 60℃; for 2.5h; | 100% |
-
-
59-48-3
2-oxoindole

-
-
91-56-5
indole-2,3-dione

-
-
6011-63-8
3-hydroxy-1,3,1',3'-tetrahydro-[3,3']biindolyl-2,2'-dione

| Conditions | Yield |
|---|---|
| With water at 20℃; for 24h; | 99% |
| In ethanol for 9h; Reflux; | 96% |
| With Sulfonic-acid-functionalized nanoporous silica In water at 140℃; for 0.5h; Mechanism; Time; Solvent; | 95% |

| Conditions | Yield |
|---|---|
| With morpholine for 16h; Knoevenagel Condensation; Reflux; | 99% |
| With pyridine; titanium(IV) isopropylate In tetrahydrofuran at 20℃; for 1h; Temperature; Knoevenagel Condensation; | 96% |
| With piperidine In ethanol for 3h; Knoevenagel condensation; Reflux; | 92% |
-
-
59-48-3
2-oxoindole

-
-
74794-91-5
3-(4-bromophenyl)-4,6-dimethoxy-1H-indole

| Conditions | Yield |
|---|---|
| With trifluoromethylsulfonic anhydride In chloroform for 0.5h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; | 99% |
| Stage #1: 2-oxoindole With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: chloromethyl methyl ether In tetrahydrofuran; N,N-dimethyl-formamide; mineral oil at 0 - 23℃; for 24h; Inert atmosphere; | 79% |
| Stage #1: 2-oxoindole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.25h; Stage #2: chloromethyl methyl ether In N,N-dimethyl-formamide at 20℃; for 3h; | 63% |
| With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; for 0.5h; | 52% |
-
-
59-48-3
2-oxoindole

-
-
26018-26-8
5-formyl-4-methyl-1H-pyrrole-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| piperidine In ethanol Heating / reflux; | 99% |
| With piperidine In ethanol Heating; | 1.98 g |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; bis[dichloro(pentamethylcyclopentadienyl)iridium(III)] at 110℃; for 15h; Inert atmosphere; Neat (no solvent); | 99% |
| With rhodium(III) chloride hydrate; triphenylphosphine; sodium hydroxide at 110℃; for 20h; Inert atmosphere; | 79% |
| With rhodium(III) chloride hydrate; triphenylphosphine; sodium hydroxide at 110 - 115℃; Sealed tube; Inert atmosphere; | 74% |
-
-
59-48-3
2-oxoindole

-
-
4663-33-6, 62668-02-4, 62839-70-7, 116127-91-4, 132014-29-0, 136981-81-2, 148616-45-9
1,3-diphenyl-3-hydroxypropene

-
-
1383192-54-8
(E)-1-(1,3-diphenylallyl)indolin-2-one

| Conditions | Yield |
|---|---|
| With perrhenic acid anhydride In dichloromethane at 20℃; for 0.166667h; | 99% |

| Conditions | Yield |
|---|---|
| With piperidine In ethanol at 20℃; for 3h; | 99% |
Oxindole Chemical Properties
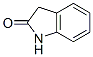
IUPAC Name:1,3-dihydroindol-2-one
Molecular Formula:C8H7NO
Molecular Weight:133.147280 g/mol
Melting Point:123-128 °C(lit.)
Boiling Point:227 °C73 mm Hg(lit.)
Flash Point:194-196°C/17mm
Insolubility:Water
EINECS:200-429-5
BRN:114692
CAS DataBase Reference:59-48-3(CAS DataBase Reference)
NIST Chemistry Reference:59-48-3(NIST)
EPA Substance Registry:System 59-48-3(EPA Substance)
Synonyms of 2-OXINDOLE(59-48-3):
OXINDOL;OXINDOLE;O-(AMINOPHENYL)ACETIC ACID LACTAM;TIMTEC-BB SBB004215;1,3-dihydro-2h-indol-2-on;1,3-Dihydro-indol-2-one;1,3-Dihydroindol-2-one;2,3-dihydroindol-2-one;Oxindole
Categories of 2-OXINDOLE(59-48-3):
blocks;IndolesOxindoles;Aromatic Phenylacetic Acids and Derivatives;Indoles and derivatives;Indoline & Oxindole;Indoles;Indole Derivatives
Oxindole Safety Profile
Hazard Codes:Xn

Risk Statements:22
22:Harmful if swallowed
Safety Statements:22-24/25
22:Do not breathe dust
24/25:Avoid contact with skin and eyes
RIDADR:2811
WGK Germany:3
RTECS:NM2080500
HazardClass:6.1(b)
PackingGroup:III
HS Code:29337900
Oxindole Specification
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
First Aid Measures of 2-OXINDOLE(59-48-3):
Ingestion:If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Skin:Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Eyes:Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View