-
Name
Paraquat dichloride
- EINECS 217-615-7
- CAS No. 1910-42-5
- Article Data36
- CAS DataBase
- Density 1.25
- Solubility soluble in water
- Melting Point > 300 °C(lit.)
- Formula C12H14Cl2N2
- Boiling Point 175oC
- Molecular Weight 257.163
- Flash Point
- Transport Information UN 2811 6.1/PG 1
- Appearance off-white powder
- Safety 22-28-36/37/39-45-60-61-28A
- Risk Codes 24/25-26-36/37/38-48/25-50/53
-
Molecular Structure
-
Hazard Symbols
 T+,
T+, N
N
- Synonyms 1,1'-Dimethyl-4,4'-bipyridiniumdichloride (6CI,7CI);1,1'-Dimethyl-4,4'-dipyridinium dichloride;AH 501;AH 501 (herbicide);Cekuquat;Cyclone Max;Dimethylviologen chloride;Galokson;Gramixel;Gramoxone;Gramoxone D;Gramoxone Max;Gramoxone S;N,N'-Dimethyl-4,4'-bipyridinium dichloride;OK 622;4,4'-Bipyridinium,1,1'-dimethyl-, chloride (1:2);
- PSA 7.76000
- LogP -4.98940
Synthetic route

| Conditions | Yield |
|---|---|
| With silver(I) chloride In water | 95% |
| With Amberlite IRA-400 |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 140℃; for 24h; | 94% |
| at 140℃; for 4h; | 2.43 g |

| Conditions | Yield |
|---|---|
| In acetonitrile at 60℃; for 12h; | 92.3% |

| Conditions | Yield |
|---|---|
| In water at 130℃; for 8h; | |
| at 20℃; for 1h; |
-
-
26985-31-9
methyl viologen cation radical

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| With ruthenium(III) In water Rate constant; | |
| With water; titanium(IV) oxide; isopropyl alcohol Equilibrium constant; Irradiation; |
-
-
1910-42-5, 79028-21-0
methyl viologen radical cation chloride

-
-
98-95-3
nitrobenzene

-
A
-
98-95-3, 12169-65-2
nitrobenzene radical anion

-
B
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| With isopropyl alcohol In water at 23℃; Equilibrium constant; Mechanism; Rate constant; Irradiation; pulse radiolytic study, effect of pH; |

-
-
45709-30-6
4-cyano-1-methylpyridinium chloride

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; titanium(IV) oxide In ethanol Irradiation; |

-
A
-
1910-42-5
paraquat dichloride

-
B
-
259886-50-5
cucurbituril

| Conditions | Yield |
|---|---|
| With Tris buffer; sodium chloride In water at 25℃; pH=7.2; Equilibrium constant; Further Variations:; Reagents; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / dimethylformamide / 1.) in an ice bath, 1 h, 2.) RT, 24 h 2: Amberlite IRA-400 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium carbonate; potassium carbonate / toluene / 6 h / 80 °C / Inert atmosphere 2: 1 h / 20 °C View Scheme |
-
-
154446-99-8
pyridin-4-yl trifluoromethanesulfonate

-
-
1692-15-5
4-pyridylboronic acid

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: bis-triphenylphosphine-palladium(II) chloride; potassium carbonate / toluene / 6 h / 80 °C / Inert atmosphere 2: 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; palladium diacetate / toluene / 6 h / 80 °C / Inert atmosphere 2: 1 h / 20 °C View Scheme |
-
-
1910-42-5, 79028-21-0
methyl viologen radical cation chloride

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| With choline chloride; urea |

| Conditions | Yield |
|---|---|
| In water | 100% |

| Conditions | Yield |
|---|---|
| In water for 72h; | 96% |

-
-
1910-42-5
paraquat dichloride

-
-
96528-49-3
(CH3NC5H4)2(2+)*CdBr2Cl2(2-)=(CH3NC5H4)2(CdBr2Cl2)

| Conditions | Yield |
|---|---|
| In not given elem. anal.; | 95% |

-
-
201230-82-2
carbon monoxide

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| With sodium acetate In methanol Pt complex was added with stirring to soln. of CH3COONa in methanol under CO; after 24 h soln. of methylviologen in methanol was added; mixt. was stirred for 1 h; filtered; washed (methanol); dried (vac.); elem. anal.; | 94% |

-
-
1910-42-5
paraquat dichloride

-
-
67994-95-0
N,N'-dimethyl-4,4'-bipyridinium dihexafluorophosphate

| Conditions | Yield |
|---|---|
| With ammonium hexafluorophosphate In water at 20℃; for 0.5h; | 93% |
| With potassium hexafluorophosphate In water at 20℃; for 2h; | 64% |
| With ammonium hexafluorophosphate In water |

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| In methanol paraquat dichloride in methanol added to a stirred soln. of SnCl4*5H2O in methanol and stirred for 15 min;; ppt. filtered, dried in air and recrystd. from MeOH/H2O (1:1) mixt.; elem. anal.;; | 90% |

| Conditions | Yield |
|---|---|
| In methanol; water Mn(NO3)2 (1 mmol) and methyl viologen dichloride (1 mmol) dissolved in H2O; stirred (30 min); soln. of Na4Re6Se8(CN)6 in methanol added; stirredovernight (room temp.); ppt. collected by filtration; washed with H2O; dried in air; elem. anal.; | 90% |

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| In water for 0.5h; Inert atmosphere; Heating; | 89% |

-
-
68088-96-0
bis(η6-1,3,5-trimethylbenzene)niobium(0)

-
-
1910-42-5
paraquat dichloride

-
-
139130-82-8
Nb(C9H12)2Cl

| Conditions | Yield |
|---|---|
| In toluene N2 or Ar atmosphere; stirring (room temp., 5 h); filtration, partial evapn., pptn. by addn. of heptane, filtration, drying (vac., room temp.); elem. anal.; | 87% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone N2-atmosphere; slow addn. of Cu-complex (in H2O) to 1.5 equiv. of bipyridinium salt (in H2O), evapn., dissoln. in H2O/NaOH, addn. of 2 equiv. ofSn-compd. (in Me2CO, pptn.); filtration, washing (H2O/Me2CO), drying (vac., overnight); elem. anal.; | 87% |

-
-
1910-42-5
paraquat dichloride

-
-
15591-62-5
1,1'-dimethyl-4,4'-bipiperidine

| Conditions | Yield |
|---|---|
| With hydrogen; rhodium In water under 760 Torr; for 5h; Product distribution; Ambient temperature; variation of catalyst, supports, reaction time; | 85% |
-
-
1910-42-5
paraquat dichloride

-
-
25128-26-1
1,1'-dimethyl-1,1'-dihydro-4,4'-bipyridyl

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium hydroxide; sodium dithionite for 2h; | 83% |
| With magnesium In acetonitrile for 48h; Ambient temperature; Yield given; | |
| controlled potential bulk electrolysis; Hg/SCE electrodes, -1.3 V; | |
| With cesium anthracene In benzene at 60℃; for 2h; |

| Conditions | Yield |
|---|---|
| With HCl In methanol to SnCl2 in degassed MeOH/HCl under inert atm. paraquat dichloride is added and stirred for 30 min;; ppt. filtered under N2 and dried in vacuo; elem. anal.;; | 82% |
-
-
1910-42-5
paraquat dichloride

-
-
7789-67-5
stannic bromide

-
-
97225-80-4
(CH3NC5H4)2(2+)*SnBr4Cl2(2-)=(CH3NC5H4)2(SnBr4Cl2)

| Conditions | Yield |
|---|---|
| In methanol paraquat dichloride in methanol added to SnBr4 in methanol and stirred for 15 min;; ppt. filtered, dried in air and recrystd. from MeOH/H2O (1:1) mixt.; elem. anal.;; | 76% |

| Conditions | Yield |
|---|---|
| In water methylviologen dichloride (0.2 mmol), KSCN (0.5 mmol) and NiCl2*6H2O (0.1 mmol) dissolved in water; soln. filtered, filtrate slowly evapd. at room temp.; crystals obtained after 7 d; elem. anal.; | 67.9% |


| Conditions | Yield |
|---|---|
| In methanol n-BuSnCl3 in methanol added dropwise to paraquat dichloride in methanol at room temp. and stirred for 20 min;; ppt. filtered, washed with methanol and dried in air; elem. anal.;; | 62% |

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| In methanol; water Heating / reflux; | 61% |

-
-
1910-42-5
paraquat dichloride

-
-
107769-57-3
(1,1'-dimethyl-4,4'-bipyridinediium) bis(2,3-quinoxalinedithiolato)zincate

| Conditions | Yield |
|---|---|
| In methanol layering of the bipyridinedium chloride in MeOH to the metal complex inMeOH and standing at room temp. for 15 h; filtn., washing with MeOH and drying (high vac.); elem. anal.; | 58% |
-
-
1421279-62-0
5-((2′-carboxy-[1,1′-biphenyl]-4-yl)methoxy)isophthalic acid

-
-
7732-18-5
water

-
-
6147-53-1
cobalt(II) diacetate tetrahydrate

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| at 140℃; for 72h; Autoclave; High pressure; | 58% |

-
-
1910-42-5
paraquat dichloride

-
-
99685-96-8, 161105-99-3, 161106-00-9, 111138-12-6, 133318-63-5, 134053-11-5, 134931-35-4, 134931-36-5, 139703-76-7, 145633-27-8, 175414-73-0, 175414-74-1, 175414-75-2, 175519-12-7, 175519-13-8, 175519-14-9, 175519-15-0, 136376-46-0, 144906-37-6, 144906-38-7, 151767-00-9, 152882-97-8, 152882-98-9, 152882-99-0, 153062-34-1, 154171-74-1, 154171-75-2, 154333-99-0, 154334-00-6, 154397-63-4, 154460-59-0, 199456-56-9, 108739-25-9, 120329-57-9, 120329-58-0
fullerene-C60

-
-
95-50-1
1,2-dichloro-benzene

-
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| Stage #1: paraquat dichloride; benzene With cesium anthracene at 60℃; for 2h; Glovebox; Inert atmosphere; Stage #2: fullerene-C60; 1,2-dichloro-benzene at 60℃; for 24h; Glovebox; Inert atmosphere; | 58% |


-
-
1910-42-5
paraquat dichloride

-
-
107769-63-1
(1,1'-dimethyl-4,4'-bipyridinediium) bis(cis-1,2-dicyano-1,2-ethenedithiolato)cadmate

| Conditions | Yield |
|---|---|
| according to A. Fernandez, H. Goerner, H. Kisch, Chem. Ber. 118 (1985) 1936; A. Fernandez, H. Kisch, ibid. 117 (1984) 3102;; elem. anal.; | 57% |

-
-
1910-42-5
paraquat dichloride

-
-
107798-06-1
(1,1'-dimethyl-4,4'-bipyridinediium) bis(cis-1,2-dicyano-1,2-ethenedithiolato)mercurate

| Conditions | Yield |
|---|---|
| according to A. Fernandez, H. Goerner, H. Kisch, Chem. Ber. 118 (1985) 1936; A. Fernandez, H. Kisch, ibid. 117 (1984) 3102;; elem. anal.; | 57% |
-
-
17084-13-8
potassium hexafluorophosphate

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-diethyl-4,4-difluoro-1,3,5,7-tetramethyl-8-(4-iodophenyl)-4-bora-3a,4a-diaza-s-indacene With N-Bromosuccinimide In dichloromethane at 20℃; for 0.5h; Darkness; Inert atmosphere; Stage #2: paraquat dichloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 0.5h; Darkness; Inert atmosphere; Stage #3: potassium hexafluorophosphate In N,N-dimethyl-formamide Inert atmosphere; | 56% |

-
-
107769-54-0
(tetrabutylammonium)2 bis(2,3-quinoxalinedithiolato)cadmate

-
-
1910-42-5
paraquat dichloride

-
-
107769-56-2
(1,1'-dimethyl-4,4'-bipyridinediium) bis(2,3-quinoxalinedithiolato)cadmate

| Conditions | Yield |
|---|---|
| In methanol layering of the bipyridinedium chloride in MeOH to the metal complex inMeOH and standing at room temp. for 15 h; filtn., washing with MeOH and drying (high vac.); elem. anal.; | 55% |
-
-
1421279-62-0
5-((2′-carboxy-[1,1′-biphenyl]-4-yl)methoxy)isophthalic acid

-
-
5743-04-4
cadmium(II) acetate dihydrate

-
-
1910-42-5
paraquat dichloride

| Conditions | Yield |
|---|---|
| In water at 130℃; for 72h; Autoclave; High pressure; | 55% |


| Conditions | Yield |
|---|---|
| With HCl In methanol equimolar amts. of Me2SnCl2 and paraquat dichloride dissolved in methanol; concd. HCl added;; soln. allowed to evaporate in desiccator, after 2 days crystals filtered and dried; elem. anal.;; | 54% |
-
-
1910-42-5
paraquat dichloride

-
-
107769-58-4
(1,1'-dimethyl-4,4'-bipyridinediium) bis(2-thioxo-1,3-dithiol-4,5-dithiolato)zincate

| Conditions | Yield |
|---|---|
| In methanol; chloroform; acetone dropwise addn. of bipyridinediium chloride in MeOH-CHCl3 to the metal complex in acetone and standing at room temp. for 15 h; filtn., washing with MeOH and drying (high vac.); elem. anal.; | 53% |
Paraquat dichloride Chemical Properties
The Molecular Structure of Methyl viologen (CAS NO.1910-42-5):

Empirical Formula: C12H14Cl2N2
Molecular Weight: 257.159
IUPAC Name: 1-methyl-4-(1-methylpyridin-1-ium-4-yl)pyridin-1-ium dichloride
Appearance: off-white powder
Nominal Mass: 256 Da
Average Mass: 257.159 Da
Monoisotopic Mass: 256.053404 Da
log P (octanol-water): -2.710 (none) EST
Water Solubility: 7.00E+05 mg/L at 20°C
Atmospheric OH Rate Constant: 2.12E-11 cm3/molecule-sec at 25°C
Storage temp: 0-6°C
Water Solubility: soluble
Stability: Stable. Incompatible with strong oxidizing agents
InChI
InChI=1/C12H14N2.2ClH/c1-13-7-3-11(4-8-13)12-5-9-14(2)10-6-12;;/h3-10H,1-2H3;2*1H/q+2;;/p-2
Smiles
c1(c2cc[n+](C)cc2)cc[n+](C)cc1.[ClH-].[ClH-]
Synonyms: Dimethyldipyridyl chloride ; Efoxon ; Dextrone x(r) ; 'lgc' (1622) ; Gramoxone ; Gramoxone(r) ; Herbaxon ; Arial gramoxone(r)
Product Categories: HERBICIDE;Functional Materials;Photochromic Compounds;Pyridinium Compounds;Viologens (Photochromic, Related Compounds);PA - PENPesticides&Metabolites;500 Series Drinking Water Methods;Alpha sort;EPA;Herbicides;Method 549Pesticides&Metabolites;N-PAlphabetic;P;Quaternary amonium salts
Paraquat dichloride History
Methyl viologen (CAS NO.1910-42-5) was first produced for commercial purposes in 1961 by Syngenta,and is today among the most commonly used herbicides. The European Union allowed paraquat in 2004. Sweden, supported by Austria, Denmark, and Finland, brought the European Union commission to court. On 11 July 2007 the court annulled the directive authorising Paraquat as an active plant protection substance.
Paraquat dichloride Uses
Methyl viologen (CAS NO.1910-42-5) is used as a quaternary ammonium herbicide, it is one of the most widely used herbicides in the world. Methyl viologen (CAS NO.1910-42-5) is also often used in science to catalyze the formation of reactive oxygen species (ROS). Methyl viologen (CAS NO.1910-42-5) will under go redox cycling in vivo, being reduced by an electron donor such NADPH, before being oxidized by an electron receptor such as dioxygen to produce superoxide, a major ROS.
Paraquat dichloride Production
Methyl viologen (CAS NO.1910-42-5) is coupled with sodium in anhydrous ammonia to give 4,4'-bipyridine, which is then methylated with chloromethane to give the desired compound PARAQUAT:
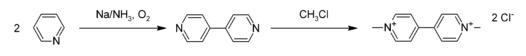
Paraquat dichloride Toxicity Data With Reference
| 1. | mmo-omi 20 ppm | MUREAV Mutation Research. 138 (1984),39. | ||
| 2. | orl-rat LD50:150 mg/kg | FMCHA2 Farm Chemicals Handbook .(Meister Publishing,Willoughy, OH.: )1983,C118. | ||
| 3. | orl-man LDLo:171 mg/kg:PUL,GIT | JTCTDW Journal of Toxicology, Clinical Toxicology. 26 (1988),35. | ||
| 4. | orl-man LDLo:1690 mg/kg:PUL,GIT | JTCTDW Journal of Toxicology, Clinical Toxicology. 26 (1988),35. | ||
| 5. | orl-man LDLo:343 mg/kg :GIT,SYS | JTCTDW Journal of Toxicology, Clinical Toxicology. 26 (1988),35. | ||
| 6. | orl-rat LD50: 100 mg/kg | IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. 10 (1979),520. | ||
| 7. | ipr-rat LD50:14,800 µg/kg | VHTODE Veterinary and Human Toxicology. 22 (1980),395. | ||
| 8. | orl-mus LD50:120 mg/kg | GEPHDP General Pharmacology. 14 (1983),541. | ||
| 9. | orl-gpg LD50:40 mg/kg | GEPHDP General Pharmacology. 14 (1983),541. |
Paraquat dichloride Consensus Reports
EPA Genetic Toxicology Program.
Paraquat dichloride Safety Profile
Poison by ingestion and intraperitoneal routes. Mutation data reported. Human systemic effects: changes in structure or function of esophagus, diarrhea, edema, fibrosis of lung, fibrosis, focal (pneumoconiosis), hemorrhage, jaundice, renal damage, renal function tests depressed, respiratory depression, ulceration or bleeding from stomach, vomiting. Death from anoxia may result. When heated to decomposition it emits toxic fumes of NOx. See also PARAQUAT DICHLORIDE. Pure paraquat ingested is highly toxic to mammals and humans potentially leading to acute respiratory distress syndrome (ARDS), and there are no specific antidotes. Even a single swig, immediately spat out, can cause death from fibrous tissue developing in the lungs leading to asphyxiation.
Hazard Codes:  T+
T+ N
N
Risk Statements: 24/25-26-36/37/38-48/25-50/53
R24/25: Toxic in contact with skin and if swallowed
R26: Very toxic by inhalation
R36/37/38: Irritating to eyes, respiratory system and skin
R48/25: Toxic: danger of serious damage to health by prolonged exposure if swallowed
R50/53: Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
Safety Statements: 22-28-36/37/39-45-60-61-28A
S22: Do not breathe dust
S28: After contact with skin, wash immediately with plenty of soap-suds
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S60: This material and its container must be disposed of as hazardous waste
S61: Avoid release to the environment. Refer to special instructions / safety data sheets
S28: After contact with skin, wash immediately with plenty of soap-suds
RIDADR: UN 2811 6.1/PG 1
WGK Germany: 3
RTECS: DW2275000
HazardClass: 6.1(a)
PackingGroup: II
Paraquat dichloride Standards and Recommendations
OSHA PEL: Respirable Dust: TWA 0.1 mg/m3 (skin)
ACGIH TLV: TWA 0.1 mg/m3
Paraquat dichloride Analytical Methods
For occupational chemical analysis use NIOSH: Paraquat 5003.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View