-
Name
Phosphorus pentasulfide
- EINECS 215-242-4
- CAS No. 1314-80-3
- Density 2.09 g/cm3
- Solubility hydrolyses in water, 0.222 g / 100g CS2 (at 17 °C), insoluble in C6H6, insoluble in hot xylene, insoluble in hot anisole
- Melting Point 288 °C (561 K)
- Formula P4S10
- Boiling Point 514 °C (787 K)
- Molecular Weight 444.54
- Flash Point
- Transport Information UN 1340
- Appearance yellow solid
- Safety 61
- Risk Codes 11-20/22-29-50
-
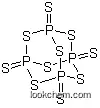
Molecular Structure
-
Hazard Symbols
 F,
F, Xn,
Xn, N
N
- Synonyms Phosphorus sulfide(Tech Grade);Phosphorus Pentasulphide;Phosphoric sulfide;Phosphorus pentasulfide, free from yellow or white phosphorus (DOT);Sulfur phosphide;Rcra waste number U189;Pentasulfure de phosphore (french);Phosphorus persulfide;Sirnik fosforecny (czech);Thiophosphoric anhydride;
- PSA 221.94000
- LogP 4.96340
Phosphorus pentasulfide Consensus Reports
Phosphorus pentasulfide Standards and Recommendations
ACGIH TLV: TWA 1 mg/m3; STEL 3 mg/m3
DFG MAK: 1 mg/m3
DOT Classification: 4.3; Label: Dangerous When Wet
Phosphorus pentasulfide Specification
The Phosphorus pentasulfide, with the CAS registry number 1314-80-3, is also known as Pentasulfure de phosphore. It belongs to the product category of Inorganics. Its EINECS registry number is 215-242-4. This chemical's molecular formula is P4S10 and molecular weight is 444.555. Its systematic name is called tricyclo[3.3.1.13,7]tetraphosphathiane 1,3,5,7-tetrasulfide. What's more, this chemical's classification code is Skin / Eye Irritant.
Physical properties of Phosphorus pentasulfide: (1)#H bond acceptors: 0; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 0; (4)Polar Surface Area: 319.4 Å2; (5)Index of Refraction: 2.029; (6)Molar Refractivity: 98.95 cm3; (7)Molar Volume: 194.1 cm3; (8)Surface Tension: 175.7 dyne/cm; (9)Density: 2.28 g/cm3.
Preparation of Phosphorus pentasulfide: this chemical is obtained by the reaction of liquid white phosphorus (P4) with sulfur above 300 °C. The first synthesis of P4S10 by Berzelius in 1843 was by this method. Alternatively, P4S10 can be formed by reacting elemental sulfur or pyrite, FeS2, with ferrophosphorus, impure Fe2P (a byproduct of P4 production from phosphate rock):
4 Fe2P + 18 S → P4S10 + 8 FeS
4 Fe2P + 18 FeS2 + heat → P4S10 + 26 FeS
Uses of Phosphorus pentasulfide: The compound is mainly converted to other derivatives for use as lubrication additives such zinc dithiophosphates. It is also used in the production of pesticides such as Parathion and Malathion. It is also a component of some amorphous solid electrolytes (e.g. Li2S-P2S5) for some types of lithium batteries. Phosphorus pentasulfide is a dual-use material, as it can be used for manufacture of the VX nerve agent.
When you are using this chemical, please be cautious about it as the following:
This chemical may catch fire in contact with air and only need brief contact with an ignition source which has a very low flash point or evolve highly flammable gases in contact with water. Meanwhile. this chemical may cause damage to health and may present an immediate or delayed danger to one or more components of the environment. On the other hand, it is highly flammable and is harmful by inhalation and if swallowed. Contact with water, it will liberate toxic gas. You should avoid releasing it to the environment.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: P12(=S)SP3(=S)SP(=S)(S1)SP(=S)(S2)S3
(2)InChI: InChI=1S/P4S10/c5-1-9-2(6)12-3(7,10-1)14-4(8,11-1)13-2
(3)InChIKey: CYQAYERJWZKYML-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 3160mg/kg (3160mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | National Technical Information Service. Vol. OTS0571217, |
| rat | LD50 | oral | 389mg/kg (389mg/kg) | "Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku," Marhold, J.V., Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha, Czechoslovakia, 1972Vol. -, Pg. 16, 1972. |
Related Products
- Phosphorus
- Phosphorus azide difluoride
- PHOSPHORUS AZIDE DIFLUORIDE-BORANE
- Phosphorus bromide difluoride
- Phosphorus cyanide
- Phosphorus heptasulfide
- Phosphorus Nitride
- Phosphorus oxybromide
- Phosphorus oxychloride
- PHOSPHORUS PENTABROMIDE
- 13148-05-5
- 13148-22-6
- 13148-43-1
- 1314-84-7
- 131-48-6
- 1314-87-0
- 131488-15-8
- 13149-00-3
- 1314-91-6
- 1314-96-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View