-
Name
PROMETHAZINE
- EINECS
- CAS No. 60-87-7
- Article Data29
- CAS DataBase
- Density 1.131
- Solubility 383.9ug/L(22.5 oC)
- Melting Point 60
- Formula C17H20 N2 S
- Boiling Point 190-192oC at 3 mm Hg
- Molecular Weight 284.425
- Flash Point
- Transport Information
- Appearance
- Safety Poison by ingestion, intravenous, intramuscular, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: pupillary dilation, wakefulness, hallucinations, and distorted perceptions. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A severe eye irritant. When heated to decomposition it emits very toxic fumes of NOx and SOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Phenothiazine,10-[2-(dimethylamino)propyl]- (8CI);(2-Dimethylamino-2-methyl)ethyl-N-dibenzoparathiazine; (?à)-Promethazine;10-[2-(Dimethylamino)propyl]phenothiazine; Dimapp; Diphergan; NSC 30321;Proazamine; Procit; Prometazin; Promethazine; Protazine; Prothazin; RP 3277;Vallergine
- PSA 31.78000
- LogP 4.30440
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane; water Reagent/catalyst; | 96% |
| With sodium hydroxide In diethyl ether Purification / work up; | 90% |
-
-
124-40-3
dimethyl amine

-
-
15375-56-1
1-(10H-phenothiazin-10-yl)acetone

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In N,N-dimethyl acetamide at 50℃; | 75% |
-
-
92-84-2
10H-phenothiazine

-
-
17256-39-2
2-(dimethyl-amino)-1-methylethylchloride hydrochloride

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Aliquat 336 at 80℃; for 1h; | 71% |
-
-
92-84-2
10H-phenothiazine

-
-
108-14-5
2-chloro-1-dimethylaminopropane

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| racemat; | |
| racemat; |
-
-
92-84-2
10H-phenothiazine

-
-
108-14-5
2-chloro-1-dimethylaminopropane

-
A
-
303-14-0
isopromethazine

-
B
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| racemat; |

-
-
92-84-2
10H-phenothiazine

-
-
53309-35-6
(2-chloro-1-methyl-ethyl)-dimethyl-amine

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| (i) NaNH2, toluene, (ii) /BRN= 505990/; Multistep reaction; |
-
-
72332-06-0
2-<2-(dimethylamino)propyl> phenothiazine-10-carboxylate

-
A
-
92-84-2
10H-phenothiazine

-
B
-
303-14-0
isopromethazine

-
C
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| at 180 - 220℃; for 2.5h; | A 840 mg B 148 mg C 454 mg |
| at 180 - 220℃; for 2.5h; Mechanism; | A 840 mg B 148 mg C 454 mg |

-
-
38878-40-9
promethazine cation

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With hydrogen sulfite In water Rate constant; Irradiation; variation of pH; |

| Conditions | Yield |
|---|---|
| With water Product distribution; Mechanism; pH 7; var. phenothiazine cation radicals, var. buffers; |

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate; N,N-dimethyl-formamide racemat; |

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethyl ether; water Purification / work up; |
-
-
60-87-7, 67253-23-0, 73745-50-3, 92998-17-9
promethazine cation radical

-
A
-
7640-51-9
Promethazine 5-sulfoxide

-
B
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With water In sulfuric acid at 24.84℃; Kinetics; Concentration; pH-value; |

| Conditions | Yield |
|---|---|
| With formic acid at 20℃; | 0.1023 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium iodide; sodium hydrogencarbonate / 80 °C 2: sodium tris(acetoxy)borohydride / N,N-dimethyl acetamide / 50 °C View Scheme |
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With isopropyl alcohol; Cysteamine; acetone In water Rate constant; Product distribution; Irradiation; pH=3; pulse radiolysis; other thiol reagents; | 100% |
| With halothane peroxyl radical In various solvent(s) Rate constant; Ambient temperature; Irradiation; pH=7; pulse radiolysis; different PZ concentrations; | 69 % Spectr. |

| Conditions | Yield |
|---|---|
| In water at 25℃; for 24h; Darkness; | 100% |
-
-
1283712-39-9
1,7-gluconamidoheptanoic acid

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
-
1283712-40-2
Glu6P

| Conditions | Yield |
|---|---|
| In water at 25℃; for 24h; Darkness; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water for 2h; | 95% |
| With dihydrogen peroxide In ethanol | |
| With n-C4F9; perfluoro-cis-2,3-dialkyloxaziridine; n-C3F7 In various solvent(s) Yield given; |

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| In dichloromethane at 70℃; for 1h; | 94% |
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
-
37707-23-6
N-Demethylpromethazine

| Conditions | Yield |
|---|---|
| Stage #1: 10-[2-(dimethylamino)propyl]phenothiazine With carbonochloridic acid 1-chloro-ethyl ester In 1,2-dichloro-ethane at 0 - 115℃; for 43h; Stage #2: With methanol at 75℃; for 18h; Reagent/catalyst; Temperature; | 92% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; [Ir[2-(2,4-difluorophenyl)-5-trifluoromethylpyridine]2(4,4′-di-t-Bu-2,2′-bipyridine)]PF6; tetra(n-butyl)ammonium hydrogensulfate In water; acetonitrile for 72h; Irradiation; regioselective reaction; | 92% |
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
-
13582-96-2, 13582-97-3, 13754-56-8
9,9-Dioxopromethazin

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate; sodium carbonate; triphenylphosphine In toluene at 80℃; for 18h; Reagent/catalyst; Temperature; Green chemistry; | 90.2% |
-
-
41144-01-8
(+/-)-citronellyl tosylate

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| Stage #1: 10-[2-(dimethylamino)propyl]phenothiazine With sodium hydroxide In diethyl ether Stage #2: (+/-)-citronellyl tosylate In acetonitrile at 40℃; for 168h; Darkness; | 83% |

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| In dichloromethane at 70℃; for 1h; | 48% |
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
-
21085-72-3
1-bromo-2,3,4-tri-O-acetyl-α-D-glucuronic acid methyl ester

-
-
145823-18-3
((2R,3R,4S,5S,6S)-6-Carboxy-3,4,5-trihydroxy-tetrahydro-pyran-2-yl)-dimethyl-(1-methyl-2-phenothiazin-10-yl-ethyl)-ammonium; chloride

| Conditions | Yield |
|---|---|
| With XAD-2 ion-exchange resin; sodium hydrogencarbonate In water; benzene for 96h; Ambient temperature; | 18% |
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
-
312488-15-6
10-(1-propenyl)phenothiazine
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide |
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In ethanol |
-
-
69884-58-8
trichloromethyl peroxyl

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
A
-
94089-33-5
Trichlormethylhydroperoxid

| Conditions | Yield |
|---|---|
| With water at 20℃; Rate constant; kinetic isotope effect; |

-
-
60-80-0
1,5-Dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
A
-
60-80-0
antipyrine

| Conditions | Yield |
|---|---|
| Rate constant; |

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
-
76069-04-0
phenothiazine radical cation

| Conditions | Yield |
|---|---|
| With trichloromethylperoxyl Rate constant; pH 7, pH 6, absolute rate constants; | |
| With CH3Cl2O2 Rate constant; pH 7, pH 6, absolute rate constants; | |
| With chloromethylperoxy radical Rate constant; pH 7, pH 6, absolute rate constants; |
-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
-
60-87-7, 67253-23-0, 73745-50-3, 92998-17-9
promethazine cation radical

| Conditions | Yield |
|---|---|
| With perchloric acid; Manganase-(III) solution In water at 7℃; Kinetics; Mechanism; Rate constant; activation parameters; | |
| With trifluoroacetic acid; dibenzoyl peroxide In toluene | |
| With air In sulfuric acid at 24.84℃; |

-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
A
-
60-87-7, 67253-23-0, 73745-50-3, 92998-17-9
promethazine cation radical

-
-
7295-85-4
catechin

| Conditions | Yield |
|---|---|
| at 20℃; Equilibrium constant; pH=3.0; | |
| at 20℃; Rate constant; Equilibrium constant; pH=3.0; |


-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
A
-
60-87-7, 67253-23-0, 73745-50-3, 92998-17-9
promethazine cation radical

-
B
-
970-73-0, 970-74-1, 1617-55-6, 3371-27-5, 13425-13-3, 136892-45-0
(+)-gallocatechin

| Conditions | Yield |
|---|---|
| at 20℃; Equilibrium constant; pH=3.0; | |
| at 20℃; Rate constant; Equilibrium constant; pH=3.0; |


-
-
60-87-7
10-[2-(dimethylamino)propyl]phenothiazine

-
A
-
60-87-7, 67253-23-0, 73745-50-3, 92998-17-9
promethazine cation radical

-
B
-
520-26-3
hesperidin

| Conditions | Yield |
|---|---|
| at 20℃; Equilibrium constant; pH=3.0; | |
| at 20℃; Rate constant; Equilibrium constant; pH=3; |

Promethazine Chemical Properties
Chemical Name: Promethazine
IUPAC NAME: N,N-Dimethyl-1-phenothiazin-10-ylpropan-2-amine
CAS No.: 60-87-7
EINECS: 200-489-2
Molecular Formula: C17H20N2S
Molecular Weight: 284.42 g/mol
Density: 1.131 g/cm3
Flash Point: 198 °C
Boiling Point: 403.7 °C at 760 mmHg
Following is the structure of 10-(2-(Dimethylamino)propyl)pheno-thiazine (CAS No.60-87-7):
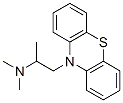
The chemical synonymous of 10-(2-(Dimethylamino)propyl)pheno-thiazine (CAS No.60-87-7) are (2-Dimethylamino-2-Methyl)Ethyl-N-Dibenzoparathiazine ; (Dimethylamino-2-Propyl-10-Phenothiazine Hydrochloride ; 10-(2-(Dimethylamino)-2-Methylethyl)Phenothiazine ; 10-[2-(Dimethylamino)Propyl]Phenothiazine ; 10h-Phenothiazine-10-Ethanamine, N,N,Alpha-Trimethyl-
Promethazine Toxicity Data With Reference
| 1. | eye-rbt 100 mg SEV | FCTOD7 Food and Chemical Toxicology. 20 (1982),573. | ||
| 2. | eye-rbt 100 mg/4S rns MLD | FCTOD7 Food and Chemical Toxicology. 20 (1982),573. | ||
| 3. | dni-hmn:fbr 80 nmol/L | DNSYAG Diseases of the Nervous System. 29 (1968),829. | ||
| 4. | otr-ham:emb 10 mg/L | ENMUDM Environmental Mutagenesis. 8 (Suppl 6)(1986),4. | ||
| 5. | msc-ham:lng 10 mg/L | SHIGAZ Shigaku. Ondotology. 70 (1983),943. | ||
| 6. | skn-cld TDLo:13 mg/kg:EYE,PSY | CMAJAX Canadian Medical Association Journal. 130 (1984),1460. | ||
| 7. | ipr-rat LDLo:140 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 1 (1959),156. | ||
| 8. | scu-rat LD50:700 mg/kg | CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. 144 (1950),887. | ||
| 9. | ivn-rat LD50:45 mg/kg | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 120 (1959),450. | ||
| 10. | ims-rat LD50:169 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 18 (1971),185. | ||
| 11. | & |
Promethazine Safety Profile
Poison by ingestion, intravenous, intramuscular, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: pupillary dilation, wakefulness, hallucinations, and distorted perceptions. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A severe eye irritant. When heated to decomposition it emits very toxic fumes of NOx and SOx.
Related Products
- Promethazine
- Promethazine hydrochloride
- Promethazine theoclate
- 60878-87-7
- 608-80-0
- 60882-70-4
- 60886-80-8
- 6089-04-9
- 6089-09-4
- 60893-02-9
- 60894-49-7
- 60894-96-4
- 60899-33-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View