-
Name
ETHYL ISOPROPYL ETHER
- EINECS 210-900-7
- CAS No. 625-54-7
- Article Data33
- CAS DataBase
- Density 0.748 g/cm3
- Solubility 24.09g/L(temperature not stated)
- Melting Point -117.26°C (estimate)
- Formula C5H12O
- Boiling Point 63.5 °C at 760 mmHg
- Molecular Weight 88.1497
- Flash Point
- Transport Information UN 2615
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ether,ethyl isopropyl (6CI,7CI,8CI);2-Ethoxypropane;Ethyl iso-propyl ether;Ethylisopropyl ether;Isopropyl ethyl ether;
- PSA 9.23000
- LogP 1.43130
Propane, 2-ethoxy- Specification
This chemical is called Propane, 2-ethoxy-, and its systematic name is 2-Ethoxypropane. With the molecular formula of C5H12O, its molecular weight is 88.15. The CAS registry number of the chemical is 625-54-7. In addition, this chemical should be put into cold storage.
Other characteristics of Propane, 2-ethoxy- can be summarised as followings: (1)ACD/LogP: 1.33; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.33; (4)ACD/LogD (pH 7.4): 1.33; (5)ACD/BCF (pH 5.5): 6.02; (6)ACD/BCF (pH 7.4): 6.02; (7)ACD/KOC (pH 5.5): 125.83; (8)ACD/KOC (pH 7.4): 125.83; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 9.23 Å2; (13)Index of Refraction: 1.374; (14)Molar Refractivity: 26.91 cm3; (15)Molar Volume: 117.8 cm3; (16)Polarizability: 10.67×10-24cm3; (17)Surface Tension: 19.6 dyne/cm; (18)Density: 0.748 g/cm3; (19)Enthalpy of Vaporization: 29.3 kJ/mol; (20)Boiling Point: 63.5 °C at 760 mmHg; (21)Vapour Pressure: 183 mmHg at 25°C.
Production method of this chemical: The Propane, 2-ethoxy- could be obtained by the reactant of Acetic acid isopropyl ester. This reaction needs the reagents of Trichlorosilane, Hydrogen chloride. The yield is 98 %. In addition, this reaction should be taken at the temperature of 60 °C. The other condition is irradiation.
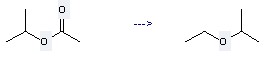
Uses of this chemical: The Propane, 2-ethoxy- could react with Triethylsilane, and obtain the Ethoxy-triethyl-silane and Propane. This reaction needs the Catalyst of Colloidal Ni. The yield is 90 %. In addition, this reaction should be taken for 1 hour at the temperature of 100 to 120 °C.
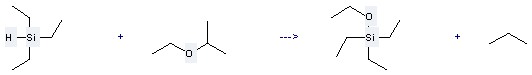
You can still convert the following datas into molecular structure:
1.SMILES: O(C(C)C)CC
2.InChI: InChI=1/C5H12O/c1-4-6-5(2)3/h5H,4H2,1-3H3
3.InChIKey: XSJVWZAETSBXKU-UHFFFAOYAW
4.Std. InChI: InChI=1S/C5H12O/c1-4-6-5(2)3/h5H,4H2,1-3H3
5.Std. InChIKey: XSJVWZAETSBXKU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC50 | inhalation | 220gm/m3/15M (220000mg/m3) | BEHAVIORAL: GENERAL ANESTHETIC | Anesthesiology. Vol. 11, Pg. 455, 1950. Link to PubMed |
Related Products
- Propane
- Propane 1,2-Cyclic Sulfate
- Propane diisothiourea dihydrobromide
- Propane, 1,3-dibromo-1,1-difluoro-
- Propane, 1,3-difluoro-
- Propane, 1,3-dinitro-
- Propane, 1-fluoro-
- Propane, 1-iodo-3-[(2-methoxyethoxy)methoxy]-
- Propane, 2,2-difluoro-
- Propane, 2-ethoxy-
- 62-55-5
- 625-55-8
- 62555-84-4
- 625-56-9
- 625-57-0
- 62558-08-1
- 625-59-2
- 62559-23-3
- 625-60-5
- 62561-03-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View