-
Name
Propane
- EINECS 200-827-9
- CAS No. 74-98-6
- Article Data1353
- CAS DataBase
- Density 0.565 g/cm3
- Solubility
- Melting Point -188 °C(lit.)
- Formula C3H8
- Boiling Point -42.1 °C
- Molecular Weight 44.0965
- Flash Point -104 °C
- Transport Information UN 1978 2.1
- Appearance colourless odourless gas
- Safety 9-16
- Risk Codes 12
-
Molecular Structure
-
Hazard Symbols
 F+
F+
- Synonyms n-Propane;Dimethylmethane;HC 290;LPG;Liquefied petroleum gas;Propyl hydride;Purifrigor P 2;Purifrigor P 3;R 280;
- PSA 0.00000
- LogP 1.41630
Synthetic route
-
-
187737-37-7
propene

-
-
74-98-6
propane

| Conditions | Yield |
|---|---|
| With para-hydrogen under 5414.51 Torr; Flow reactor; | 100% |
| With hydrogen; nickel at 40℃; Thermodynamic data; Ea, various catalysts; | |
| With hydrogen at 151.9 - 326.9℃; under 100 Torr; Kinetics; Ir(1.1.1)-surface; pc-C3H6 2.0 torr; kapp0, Eapp; |
-
-
201230-82-2
carbon monoxide

-
A
-
34557-54-5
methane

-
B
-
74-84-0
ethane

-
C
-
74-98-6
propane

-
D
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| With hydrogen; nickel at 329.9℃; nickel powder, prepared by evaporation-condensation; Yields of byproduct given; | A 98% B n/a C n/a D n/a E n/a |


| Conditions | Yield |
|---|---|
| With DIP-Co catalysts at 23℃; Reagent/catalyst; Inert atmosphere; Glovebox; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| In benzene-d6 Kinetics; thermolysis at 167 +/- 1°C; | A <2 B 97% |
| In benzene Irradiation (UV/VIS); photolysis in a frozen benzene soln.; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) 150°C, 60 h; further products; | A 91% B 5% C 95% |

-
-
56-23-5
tetrachloromethane

-
-
80937-33-3
oxygen

-
-
1071-39-2
diisopropylmercury

-
A
-
74-98-6
propane

-
B
-
75-29-6
isopropyl chloride

-
C
-
67-66-3
chloroform

-
D
-
30615-19-1
isopropylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) 20°C, 96 h; further products; | A 4% B 48% C 30% D 95% E 5% |

-
-
617-86-7
triethylsilane

-
-
625-54-7
2-butyl ethyl ether

-
A
-
74-98-6
propane

-
B
-
597-67-1
ethoxytriethylsilane

| Conditions | Yield |
|---|---|
| nickel at 100 - 120℃; for 1h; | A n/a B 90% |

-
-
617-86-7
triethylsilane

-
-
1860-27-1
butyl isopropyl ether

-
A
-
74-98-6
propane

-
B
-
2751-87-3
n-butoxytriethylsilane

| Conditions | Yield |
|---|---|
| nickel at 100 - 120℃; for 1h; | A n/a B 90% |


| Conditions | Yield |
|---|---|
| With triethylamine In water at 20℃; for 2h; Inert atmosphere; Irradiation; | 90% |
| Co(BF4)2(bipy)n Product distribution; Electrochemical reaction; |

-
-
7732-18-5
water

-
A
-
34557-54-5
methane

-
B
-
74-84-0
ethane

-
C
-
74-98-6
propane

-
D
-
74-85-1
ethene

-
E
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| In neat (no solvent) hydrolysis in water vapor (11.0E2 Nm**2); | A 0.24% B 6.77% C 0.14% D 2.54% E 89.5% |


| Conditions | Yield |
|---|---|
| With hydrogen In further solvent(s) High Pressure; in perhydrocumol at 100 at;; | A 89% B n/a |
| With H2 In further solvent(s) High Pressure; in perhydrocumol at 100 at;; | A 89% B n/a |
-
-
21373-88-6, 16920-54-0
(PPh3)3CoH(N2)

-
-
4346-18-3
phenyl butanoate

-
A
-
53729-69-4, 21329-67-9
HCo(CO)(P(C6H5)3)3

-
B
-
91583-66-3
phenoxotris(triphenylphosphine)cobalt(I)

-
C
-
74-98-6
propane

-
D
-
109-21-7
butyl butyrate

-
E
-
7727-37-9
nitrogen

| Conditions | Yield |
|---|---|
| In toluene byproducts: H2; n-PrCO2Ph added to CoH(N2)(PPh3)3 in toluene in vac., reacted for 1 day at room temp.; liquid phase analysed by GLC; hexane added, ppt. filtered, washed with hexane, dried in vac., recrystd. from C6H6-hexane; | A n/a B 60% C 12% D 38% E 89% |

-
-
67-56-1
methanol

-
A
-
187737-37-7
propene

-
B
-
34557-54-5
methane

-
C
-
74-84-0
ethane

-
D
-
74-98-6
propane

-
E
-
74-85-1
ethene

-
F
-
106-97-8
n-butane

| Conditions | Yield |
|---|---|
| Ni-SAPO-34 at 450℃; under 760 Torr; for 1h; Product distribution; var. temp.; | A 5.26% B 1.5% C 1.02% D 0.15% E 88.04% F 0.03% |
| molecular sieve In gas at 300℃; Product distribution; other temperatures; other products; | |
| With hydrogen; proton-type ZSM-5 at 330℃; under 1050.11 Torr; Product distribution / selectivity; Gas phase; |

-
-
463-82-1
2,2-dimethylpropane

-
A
-
34557-54-5
methane

-
B
-
74-84-0
ethane

-
C
-
74-98-6
propane

-
D
-
75-28-5
Isobutane

-
E
-
78-78-4
methylbutane

| Conditions | Yield |
|---|---|
| With hydrogen; platinum at 243℃; Product distribution; further reaction temperatures, catalysts; | A 2.7% B 1.5% C 1.5% D 6.7% E 87.8% |
| at 304℃; Product distribution; also from n-butane, other products,other temperatures, other catalysts; | A 7% B 1.7% C 2.4% D 15.6% E 73.3% |
| With hydrogen; NaY-500; palladium at 216℃; Product distribution; Kinetics; Thermodynamic data; other catalysts, other temperatures; activation energies; | |
| With hydrogen In neat (no solvent) at 275℃; under 1225.5 Torr; Reagent/catalyst; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane at 159.84℃; under 60006 Torr; for 24h; Autoclave; | A 87% B 9% |
| With [Ru(OH2)3(4'-phenyl-2,2':6',2''-terpy)](OTf)2; hydrogen; ortho-tungstic acid In water at 250℃; under 41254.1 Torr; for 24h; | A 39 %Chromat. B 52 %Chromat. |
| With hydrogen In water at 200℃; under 18751.9 Torr; for 16h; Autoclave; |

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
74-98-6
propane

-
E
-
74-85-1
ethene

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 60°C (15 h); further product: cyclobutane; | A 85.9% B 3.4% C 3.6% D 1% E 5.7% |
| In toluene thermal decompn. at 95°C (15 h); further product: cyclobutane; | A 58.7% B 2.5% C 2.5% D 1% E 36.3% |

-
-
3531-43-9
tetraisobutyl stannane

-
A
-
74-98-6
propane

-
B
-
75-28-5
Isobutane

-
C
-
115-11-7
isobutene

-
D
-
106-97-8
n-butane

| Conditions | Yield |
|---|---|
| With hydrogen byproducts: C5 hydrocarbons, paraffin; 20 h, 300-310°C, 100 atm; | A 1.6% B 85.7% C 2.5% D 3.1% |
| With H2 byproducts: C5 hydrocarbons, paraffin; 20 h, 300-310°C, 100 atm; | A 1.6% B 85.7% C 2.5% D 3.1% |


| Conditions | Yield |
|---|---|
| With methanol; toluene-4-sulfonic acid at 25℃; for 12h; Time; Sealed tube; Inert atmosphere; UV-irradiation; | A 85% B 11% |
| With methanol; 5% Pd/TiO2; toluene-4-sulfonic acid at 25℃; for 12h; Inert atmosphere; UV-irradiation; |
-
-
201230-82-2
carbon monoxide

-
A
-
187737-37-7
propene

-
B
-
34557-54-5
methane

-
C
-
74-84-0
ethane

-
D
-
74-98-6
propane

| Conditions | Yield |
|---|---|
| With hydrogen; nickel at 473℃; under 159.8 Torr; | A 8% B 84% C 4% D 4% |
| With hydrogen; cobalt-manganese oxide at 190℃; under 4500.4 Torr; Further byproducts given. Yields of byproduct given; | |
| With hydrogen; TiC Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
106-97-8
n-butane

-
A
-
187737-37-7
propene

-
B
-
34557-54-5
methane

-
C
-
74-84-0
ethane

-
D
-
74-98-6
propane

-
E
-
74-85-1
ethene

| Conditions | Yield |
|---|---|
| at 570 - 630℃; Kinetics; | A 68% B 83% C 20% D 4% E 30% |
| ferrerite zeolite at 650℃; |

-
-
106-31-0
butanoic acid anhydride

-
-
21373-88-6, 16920-54-0
(PPh3)3CoH(N2)

-
A
-
99668-71-0
Co(OCO-n-C3H7)

-
B
-
74-98-6
propane

-
C
-
109-21-7
butyl butyrate

-
D
-
7727-37-9
nitrogen

-
E
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| In toluene in toluene at room temp. for 1 day; | A n/a B 16% C 30% D 81% E 13% |

-
-
74-96-4
ethyl bromide

-
-
31387-22-1
dimethylnickel{1,2-bis(diphenylphosphino)ethane}

-
A
-
14647-21-3
dibromo[1,2-bis(diphenylphosphino)ethane]nickel(II)

-
B
-
34557-54-5
methane

-
C
-
74-84-0
ethane

-
D
-
74-98-6
propane

-
E
-
74-85-1
ethene

| Conditions | Yield |
|---|---|
| In toluene EtBr added into toluene soln. of Ni complex, stirred at 35°C for48 h; evapd. in vac., crystd. from Et2O-hexane; GLC anal.; | A 48% B 78% C 70% D 10% E 33% |

-
-
67-66-3
chloroform

-
-
2386-64-3
ethylmagnesium chloride

-
A
-
74-98-6
propane

-
B
-
617-78-7
3-ethylpentane

-
C
-
109-66-0
pentane

| Conditions | Yield |
|---|---|
| With C31H37ClN3NiO2(1-)*Li(1+) In tetrahydrofuran at 25℃; for 0.333333h; Inert atmosphere; Overall yield = 93.4 %; | A 6% B 9.4% C 78% |
| With C31H37ClFeN3O2 In tetrahydrofuran at 25℃; for 0.0833333h; Inert atmosphere; |

-
-
106-31-0
butanoic acid anhydride

-
A
-
99668-71-0
Co(OCO-n-C3H7)

-
B
-
187737-37-7
propene

-
C
-
34557-54-5
methane

-
D
-
74-98-6
propane

-
E
-
107-87-9
2-Pentanone

| Conditions | Yield |
|---|---|
| In tetrahydrofuran in THF at -40-20°C; | A n/a B 7% C 6% D 2% E 76% |


| Conditions | Yield |
|---|---|
| With hydrogen; Ni-B(P-1) at 473℃; under 159.8 Torr; | A 75% B 17% C 8% |
| With hydrogen; technetium at 240℃; Product distribution; Thermodynamic data; E(a); other supporting materials of the technetium catalyst; var. temperatures; | A 81.0 % Chromat. B 17.9 % Chromat. C 1.1 % Chromat. |
| With hydrogen at 210.3℃; under 3723.56 Torr; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| With sulfuric acid In not given acidolysis with H2SO4 (residue not identified); | A 74.5% B 16.3% C 21% D 72.3% |


| Conditions | Yield |
|---|---|
| for 16h; Product distribution / selectivity; γ-Irradiation; 25 psig; | A 74% B n/a |
-
-
556-56-9
allyl iodid

-
-
12103-40-1
ReH7*2P(C6H5)3=ReH7(P(C6H5)3)2

-
A
-
74-98-6
propane

-
B
-
85335-13-3
2(C6H5)3PC3H5(1+)*ReI6(2-)=((C6H5)3PC3H5)2ReI6

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: H2, propene; under N2, allyl iodide was reacted with Re-complex with stirring in THFat room temp. for 3 h; filtered, washed with THF and Et2O, recrystd. from CH2Cl2-THF; elem. anal.; | A <1 B 74% |


| Conditions | Yield |
|---|---|
| In solid thermolysis at 110°C to propane, n-butane and an unidentified residue; | A 22.3% B 72.2% |
-
-
60542-85-0, 81131-93-3
trans-NiMe2(triethylphosphine)2

-
-
106-94-5
propyl bromide

-
-
69460-30-6, 19224-77-2
trans-dibromobis(triethylphosphine)nickel(II)

-
B
-
187737-37-7
propene

-
C
-
34557-54-5
methane

-
D
-
74-84-0
ethane

-
E
-
74-98-6
propane

| Conditions | Yield |
|---|---|
| In toluene propyl bromide added into toluene soln. of NiMe2(PEt3)2, stirred at room temp. for 12 h; evapd. in vac., crystd. from Et2O-hexane; GLC anal.; | A 71% B 127 % C 115 % D 46% E 35% |


| Conditions | Yield |
|---|---|
| In gas byproducts: H2O; in gaseous phase; ion cyclotron resonance; | 100% |

| Conditions | Yield |
|---|---|
| In gas reaction in a mass spectrometer; sample pressure 4E-7 Torr; | A 100% B 100% |


| Conditions | Yield |
|---|---|
| In gas byproducts: H2O; in gaseous phase; ion cyclotron resonance; | 100% |

| Conditions | Yield |
|---|---|
| In gas in gaseous phase; ion cyclotron resonance; | 100% |
-
-
74-98-6
propane

-
-
201230-82-2
carbon monoxide

-
-
10557-71-8
4-chlorophenyltrimethylsilane

-
-
18713-58-1
1-(4-chlorophenyl)-2-methylpropan-1-one

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; carbon tetrabromide In various solvent(s) at 0℃; for 0.5h; | 97% |


| Conditions | Yield |
|---|---|
| With 2AlBr3*CBr4; bromine at -20℃; for 3h; | 96% |
| With antimony pentafluoride; 1,2-dibromomethane 1.) -78 deg C, 2 h, 2.) RT, 24 h; | 64% |
| With 2AlBr3*CBr4; bromine In various solvent(s) at -20℃; for 3h; | 48 % Turnov. |

| Conditions | Yield |
|---|---|
| In 5,5-dimethyl-1,3-cyclohexadiene | 95% |
-
-
74-98-6
propane

-
-
717139-15-6, 547695-25-0
[(η3-[2.1.1]-2,6-pyridinophane)Pt(IV)HMe2]B[3,5-(CF3)2C6H3]4

-
-
566200-10-0, 566933-21-9
[Pt(η2-C3H6)H(η3-[2.1.1]-(2,6)-pyridinophane)] tetrakis[3,5-bis(trifluoromethyl)phenyl]borate

| Conditions | Yield |
|---|---|
| In dichloromethane Kinetics; byproducts: CH4; by a react. of propane (3 M) with Pt-contg. compd. at room temp. in CH2Cl2 soln. for 8 h; NMR studies; two diastereomers; | 95% |

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; carbon tetrabromide In various solvent(s) at 0℃; for 1.5h; | 94% |
| With aluminum tri-bromide; carbon tetrabromide at 0℃; for 1.5h; Mechanism; |

| Conditions | Yield |
|---|---|
| With chromium(III) oxide; carbon dioxide at 549.9℃; Product distribution; other supporting materials for Cr2O3; also in the absence of CO2; | A 91.3% B 6.4% C 0.7% D 1.6% |
| at 500 - 600℃; Kinetics; | A 46% B 55% C 7% D 47% |
| With hydrogen-permeable palladium module and chromia-alumina catalyst (9.0 wt % Cr) at 550℃; Temperature; | A 30% B n/a C n/a D n/a |


| Conditions | Yield |
|---|---|
| In gas in gaseous phase; ion cyclotron resonance; | A 10% B 90% |


| Conditions | Yield |
|---|---|
| With oxygen; multimetal oxide catalyst at 274 - 316℃; Gas phase; | 86.1% |


| Conditions | Yield |
|---|---|
| With oxygen; multimetal oxide catalyst at 274 - 316℃; Gas phase; | 86.1% |

Propane Consensus Reports
Reported in EPA TSCA Inventory.
Propane Standards and Recommendations
OSHA PEL: TWA 1000 ppm
DFG MAK: 1000 ppm (1800 mg/m3)
DOT Classification: 2.1; Label: Flammable Gas
Propane Specification
This product is an organic compound with the formula C3H8. The systematic name of this chemical is Propane. It belongs to the product categories of Refrigerants; Organics; Burners; Labware; Chemical Synthesis; Compressed and Liquefied Gases; Synthetic Reagents. Its EINECS number is 200-827-9. With the CAS registry number 74-98-6, it is also named as Dimethylmethane. In addition, the molecular weight is 44.11. Its classification codes are: (1)Aerosol propellant; (2)Aerosol propellants; (3)Agricultural Chemical; (4)Herbicide. It should be stored in the cylinder outfit which is placed in a cool place. Moreover, it should be protected from heat and fire. It is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves and residential central heating. It is also used as refrigerant. Besides, it is mainly used as raw material in the production of ethylene and propylene by cracking, as deasphalting reagent in refinery and as solvent in desulfurization.
Physical properties of Propane are: (1)ACD/LogP: 2.24; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.236; (4)ACD/LogD (pH 7.4): 2.236; (5)ACD/BCF (pH 5.5): 29.452; (6)ACD/BCF (pH 7.4): 29.452; (7)ACD/KOC (pH 5.5): 391.926; (8)ACD/KOC (pH 7.4): 391.926; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)Index of Refraction: 1.331; (12)Molar Refractivity: 15.947 cm3; (13)Molar Volume: 78.056 cm3; (14)Polarizability: 6.322×10-24cm3; (15)Surface Tension: 14.195 dyne/cm; (16)Density: 0.565 g/cm3; (17)Enthalpy of Vaporization: 19.04 kJ/mol; (18)Vapour Pressure: 7271.002 mmHg at 25°C.
Preparation: this chemical can be prepared by ethane at the temperature of 500 - 650 °C. This reaction will need reagent CO2. This reaction will also need catalyst Cr/SO4(2-)-SiO2. The yield is about 55.2%.
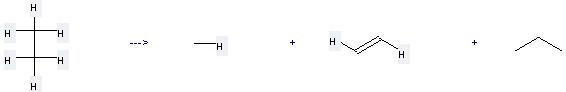
Uses of Propane: it can be used to produce 2-bromo-propane at the temperature of -20 °C. It will need reagent Br2, CBr4·2AlBr3 with the reaction time of 3 hours. The yield is about 96%.

When you are using this chemical, please be cautious about it as the following:
It is extremely flammable, so you should keep it away from sources of ignition - No smoking. You should keep the container in a well-ventilated place.
You can still convert the following datas into molecular structure:
(1)SMILES: CCC
(2)Std. InChI: InChI=1S/C3H8/c1-3-2/h3H2,1-2H3
(3)Std. InChIKey: ATUOYWHBWRKTHZ-UHFFFAOYSA-N
Related Products
- Propane
- Propane 1,2-Cyclic Sulfate
- Propane diisothiourea dihydrobromide
- Propane, 1,3-dibromo-1,1-difluoro-
- Propane, 1,3-difluoro-
- Propane, 1,3-dinitro-
- Propane, 1-fluoro-
- Propane, 1-iodo-3-[(2-methoxyethoxy)methoxy]-
- Propane, 2,2-difluoro-
- Propane, 2-ethoxy-
- 7498-65-9
- 749875-07-8
- 749875-16-9
- 74988-05-9
- 749884-42-2
- 749898-80-4
- 749902-11-2
- 7499-06-1
- 7499-07-2
- 7499-08-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View