-
Name
Rubrene
- EINECS 208-242-0
- CAS No. 517-51-1
- Article Data38
- CAS DataBase
- Density 1.176 g/cm3
- Solubility Soluble in hot toluene. Insoluble in water.
- Melting Point >315 °C(lit.)
- Formula C42H28
- Boiling Point 649 °C at 760 mmHg
- Molecular Weight 532.684
- Flash Point 351.6 °C
- Transport Information
- Appearance red crystalline powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 5,6,11,12-Tetraphenylnaphthacene;5,6,11,12-Tetraphenyltetracene;Rubren;
- PSA 0.00000
- LogP 11.81420
Synthetic route
-
-
127257-80-1
5,12-dihydro-5,12-epoxy-5,6,11,12-tetraphenylnaphthacene

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; cesium iodide In chloroform -78 deg C -> room temp.; | 88% |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride Behandlung des Reaktionsprodukts mit Kaliumacetat in Aethanol; |
-
-
56024-62-5
3-acetoxy-1,3,3-triphenylpropyne

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| beim Erhitzen; |

| Conditions | Yield |
|---|---|
| beim Erhitzen; |

| Conditions | Yield |
|---|---|
| beim Erhitzen; |
-
-
63450-98-6
1-Chlor-1.1.3-triphenyl-propin-(2)

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With quinoline at 120℃; unter vermindertem Druck; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| beim Erhitzen; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| beim Erhitzen; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With tetrahydrofuran; phenylmagnesium bromide anschliessendes Behandeln mit wss. HCl; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| beim Erhitzen; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| beim Erhitzen; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| beim Erhitzen; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene
-
-
886-65-7, 538-81-8
trans,trans-1,4-Diphenyl-1,3-butadiene

-
A
-
3029-19-4
pyrene-1-aldehyde

-
B
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With oxygen In benzene Rate constant; Irradiation; quenching of polyene triplet; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With triphenylphosphine In dichloromethane Mechanism; other aromatic radical cations; |
-
-
34507-27-2
thianthrene cation radical

-
A
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
B
-
92-85-3
Thianthrene

| Conditions | Yield |
|---|---|
| In acetonitrile emission, other cation radicals; |

-
-
1499-10-1
9,10-diphenylanthracene anion radical

-
A
-
1499-10-1
9,10-diphenylanthracene

-
B
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| In acetonitrile emission, other anion radicals; |

-
-
60-29-7
diethyl ether

-
-
98480-37-6
epoxy-5,12 phenoxy-5 triphenyl-6,11,12 dihydro-5,12 naphtacene

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
32287-37-9
5,6,11,12-tetraphenyl-5,12-dihydro-5,12-epidioxido-naphthacene

-
A
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| at 100 - 140℃; unter gruenlichgelber Luminescenz; |
-
-
32287-37-9
5,6,11,12-tetraphenyl-5,12-dihydro-5,12-epidioxido-naphthacene

-
-
71-43-2
benzene

-
A
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| at 20℃; Irradiation; |

-
-
849-01-4
1,3,3-triphenylprop-2-en-1-one

-
-
10026-13-8, 874483-75-7
phosphorus pentachloride

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| Behandlung des Reaktionsprodukts mit Kaliumacetat in Aethanol und anschliessendes Erhitzen; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| at 100 - 120℃; unter vermindertem Druck; |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With sodium hypophosphite; acetic acid; potassium iodide at 130℃; | |
| With iron; acetic acid |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With benzene | |
| With acetone |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene
-
-
882656-83-9
5r.6.11.12c-tetraphenyl-5.12-dihydro-naphthacenediol-(5.12t)

-
-
64-19-7
acetic acid

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene


-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
32287-37-9
5,6,11,12-tetraphenyl-5,12-dihydro-5,12-epidioxido-naphthacene

| Conditions | Yield |
|---|---|
| With sodium molybdate; dihydrogen peroxide; sodium dodecyl-sulfate In dichloromethane; water; butan-1-ol at 25℃; for 0.5h; | 98% |
| With bis(dimethyl-di-n-octylammonium) molybdate; dihydrogen peroxide In water; benzene at 25℃; for 0.5h; | 98% |
| With air In chloroform for 24h; Irradiation; | 97% |
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
98453-04-4
ortho-phenylene-11,12 diphenyl-6,12 oxo-5 dihydro-5,12 naphtacene

| Conditions | Yield |
|---|---|
| With hydrogenchloride In chloroform for 120h; Irradiation; | 88% |
-
-
113860-02-9
[Cp*Ru(CH3CN)3]OTf

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| In dichloromethane Heating, >4 equivalent Ru-complex.; | 85% |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane byproducts: CH3CN; at 25°C, solution was stirred for 3 h; solvent was removed by rotary evapn., solid was redissolved in CH2Cl2, chromy., washed with ether, elem. anal.; | 83% |

| Conditions | Yield |
|---|---|
| In toluene at 120 - 125℃; for 19h; Kinetics; Solvent; Temperature; Inert atmosphere; Sealed tube; Darkness; | 78% |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| In acetone refluxed; elem. anal.; | 72% |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| In acetone byproducts: MeCN; N2-atmosphere; equimolar amts., stirring for 4 h; solvent removal (reduced pressure), dissoln. in CH2Cl2, passing over Kieselguhr, pptn. on hexane addn., collection (filtration), washing (Et2O);elem. anal.; | 72% |
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 3h; | 63% |

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| In dichloromethane ratio of organic compound to coordination compound 1:2, refluxing for 23 h; chromy. (diatomaceous earth), recrystn., elem. anal.; | 51% |
-
-
930-88-1
N-methylmaleimide

-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
A
-
98453-05-5
N-methyl ethano-1,4 dihydro-1,4 tetraphenyl-5,6,11,12 naphtacene dicarboximide-13,14 (exo)

-
B
-
98524-64-2
N-methyl ethano-1,4 dihydro-1,4 tetraphenyl-5,6,11,12 naphtacene-dicarboximide-13,14 (endo)

| Conditions | Yield |
|---|---|
| In various solvent(s) for 0.5h; Heating; | A 50% B 11% |


-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| In acetone byproducts: CH3CN; solution was refluxed in acetone for 65 h; solvent was removed with a stream of N2, solid was redissolved in acetonitrile, chromy., recrystd. from acetone/ether, elem. anal.; | 40% |
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
882656-83-9
5r.6.11.12c-tetraphenyl-5.12-dihydro-naphthacenediol-(5.12t)

| Conditions | Yield |
|---|---|
| With potassium permanganate; sulfuric acid; benzene |
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
73982-26-0
5,6,11,12-tetraphenyl-5,11-dihydro-naphthacene

| Conditions | Yield |
|---|---|
| dihydrorubrene of mp: 231 degree; |
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
861095-05-8
5,6,11,12-tetraphenyl-5,12-dihydro-naphthacene-5,12-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With diethyl ether; sodium anschliessendes Behandeln mit Kohlendioxid; |
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With pyridine; osmium(VIII) oxide; benzene |
-
-
517-51-1
5,6,11,12-tetraphenylnaphthacene

-
-
127257-80-1
5,12-dihydro-5,12-epoxy-5,6,11,12-tetraphenylnaphthacene

| Conditions | Yield |
|---|---|
| With chromic acid | |
| With nitric acid | |
| With permanganate(VII) ion |
Rubrene Chemical Properties
Molecular Structure of Rubrene (CAS NO.517-51-1):
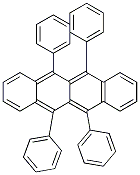
IUPAC Name: 5,6,11,12-tetraphenyltetracene
CAS: 517-51-1
Molecular Formula: C42H28
Molecular Weight: 532.67
EINECS: 208-242-0
Melting point: >315 °C(lit.)
Form: powder
Index of Refraction: 1.716
Molar Refractivity: 178.14 cm3
Molar Volume: 452.9 cm3
Polarizability: 70.62 10-24cm3
Surface Tension: 51.3 dyne/cm
Density: 1.176 g/cm3
Flash Point: 351.6 °C
Enthalpy of Vaporization: 92.21 kJ/mol
Boiling Point: 649 °C at 760 mmHg
Vapour Pressure: 5.14E-16 mmHg at 25°C
InChI
InChI=1/C42H28/c1-5-17-29(18-6-1)37-33-25-13-14-26-34(33)39(31-21-9-3-10-22-31)42-40(32-23-11-4-12-24-32)36-28-16-15-27-35(36)38(41(37)42)30-19-7-2-8-20-30/h1-28H
Smiles
c12c(c(c3c(cccc3)c1c1ccccc1)c1ccccc1)c(c1ccccc1)c1c(cccc1)c2c1ccccc1
Product Categories: Organics; Electronic Chemicals; Electroluminescence; Functional Materials; Highly Purified Reagents; Other Categories; Refined Products by Sublimation; Dark red crystal
Rubrene Uses
Rubrene (CAS NO.517-51-1) is mainly used as organic reagents and pharmaceutical intermediates.
Rubrene Safety Profile
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
F: 10-23
Rubrene Specification
Rubrene (CAS NO.517-51-1) is also named as 5,6,11,12-Tetraphenylnaphthacene;rubrene ; 5,6,11,12-tetraphenyl-naphthacen ; 5,6,11,12-tetraphenyl-naphthacene,(rubrene) ; 5,6,11,12-Tetraphenyltetracene ; Naphthacene, 5,6,11,12-tetraphenyl- ; naphthacene,5,6,11,12-tetraphenyl- and so on. It is a red crystalline powder.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View