-
Name
SILVER ARSENITE
- EINECS
- CAS No. 7784-08-9
- Density
- Solubility
- Melting Point
- Formula AsO3•3Ag
- Boiling Point
- Molecular Weight 446.53
- Flash Point
- Transport Information
- Appearance
- Safety Confirmed carcinogen. When heated to decomposition it emits toxic fumes of As.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms
- PSA 69.18000
- LogP -0.73720
Silver arsenite Chemical Properties
Molecule structure of Silver arsenite (CAS NO.7784-08-9) :
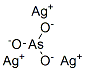
IUPAC Name: arsorous acid; silver
Molecular Weight: 449.54822 g/mol
Molecular Formula: Ag3AsH3O3
H-Bond Donor: 3
H-Bond Acceptor: 3
Exact Mass: 448.644757
MonoIsotopic Mass: 446.645094
Topological Polar Surface Area: 60.7
Heavy Atom Count: 7
Complexity: 8
Canonical SMILES: O[As](O)O.[Ag].[Ag].[Ag]
InChI: InChI=1S/3Ag.AsH3O3/c;;;2-1(3)4/h;;;2-4H
InChIKey: FQUOIMHWJGIXSS-UHFFFAOYSA-N
EINECS: 232-048-5
Silver arsenite Uses
Silver arsenite (CAS NO.7784-08-9) is used to make dyes and insecticides.
Silver arsenite Safety Profile
Confirmed carcinogen. When heated to decomposition it emits toxic fumes of As.
RIDADR: 1683
HazardClass: 6.1(a)
PackingGroup: II
Silver arsenite Standards and Recommendations
OSHA PEL: OSHA: Cancer Hazard
DOT Classification: 6.1; Label: Poison
Silver arsenite Specification
Silver arsenite (CAS NO.7784-08-9) is also called Arsenious acid, trisilver(1+) salt ; Arsenous acid, trisilver(1+) salt ; Arsenous acid, trisilver(1+) salt (9CI) ; Trisilver arsenite . Silver arsenite (CAS NO.7784-08-9) is a fine yellow powder. It is sensitive to light. Silver arsenite (CAS NO.7784-08-9) is highly toxic by ingestion and inhalation. It is water soluble. Silver arsenite has weak oxidizing or reducing powers. Redox reactions can however still occur. May be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Related Products
- Silver
- Silver (II) fluoride
- SILVER 2-AZIDO-4,6-DINITROPHENOXIDE
- SILVER 3,5-DINITROANTHRANILATE
- SILVER 3-CYANO-1-PHENYLTRIAZEN-3-IDE
- SILVER 3-METHYLISOXAZOLIN-4,5-DIONE-4-OXIMATE
- SILVER 4-NITROPHENOXIDE
- SILVER 5-AMINOTETRAZOLIDE
- Silver acetate
- Silver acetylide (dry)
- 7784-09-0
- 7784-11-4
- 7784-13-6
- 7784-16-9
- 7784-18-1
- 7784-19-2
- 7784-21-6
- 7784-22-7
- 7784-23-8
- 7784-24-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View