-
Name
Sodium pyruvate
- EINECS 204-024-4
- CAS No. 113-24-6
- Article Data22
- CAS DataBase
- Density 1.267 g/cm3
- Solubility water: 100 mg/mL
- Melting Point >300 °C(lit.)
- Formula C3H3NaO3
- Boiling Point 165 °C at 760 mmHg
- Molecular Weight 110.045
- Flash Point 54.3 °C
- Transport Information
- Appearance White to slightly yellow crystalline powder
- Safety 36/37/39-26-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Propanoicacid, 2-oxo-, sodium salt (9CI);Pyruvic acid, sodium salt (7CI,8CI);Sodiumpyruvate;Sodium a-ketopropionate;
- PSA 57.20000
- LogP -1.67470
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium formate In ethyl acetate | 97% |
| With sodium acetate In ethyl acetate | 94% |
| With sodium hydroxide; ethanol; water |

| Conditions | Yield |
|---|---|
| Stage #1: poly(methacrylic acid) With ozone In methanol; dichloromethane at -78℃; Stage #2: With dimethylsulfide In methanol; dichloromethane at -78 - 20℃; Stage #3: With sodium hydroxide In water | 92% |
-
-
97-64-3, 2676-33-7
ethyl 2-hydroxypropionate

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-hydroxypropionate With 9-azabicyclo<3.3.1>nonane-N-oxyl; dihydrogen peroxide In ethyl acetate at 10 - 40℃; for 3h; Stage #2: With sodium carbonate In water; ethyl acetate at 20℃; for 1h; pH=3 - 4; Reagent/catalyst; Temperature; | 55% |
-
-
56-41-7
L-alanin

-
-
114-76-1
sodium phenylphyruvate

-
A
-
150-30-1
Phenylalanine

-
B
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With acetate buffer; N(1+)C5His2C16; PL(1+)2C16; copper(II) perchlorate In water at 30℃; for 48h; Kinetics; |

-
-
21593-77-1, 49621-03-6
S-allyl cysteine

-
A
-
2179-57-9
diallyl disulphide

-
B
-
539-86-6
allicin

-
C
-
29418-05-1
bis-2-propenyl thiosulfonate

-
D
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With nitrogen(II) oxide In water for 24h; Ambient temperature; Yield given. Yields of byproduct given; |

-
-
44300-97-2
sodium enol pyruvate

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; iodine; potassium iodide In water at 25℃; Rate constant; Equilibrium constant; other base as reagents; | |
| With phosphate buffer; iodine; potassium iodide at 11℃; Equilibrium constant; Further Variations:; Temperatures; enolization; |
-
-
43165-43-1
sodium pyruvate hydrate

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 25℃; Rate constant; Equilibrium constant; other bases or acids reagents; | |
| With phosphate buffer; iodine; potassium iodide at 11℃; Equilibrium constant; Further Variations:; Temperatures; hydration; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; lead(II) nitrate In water Product distribution; modified platinum electrode by lead ad-atoms, effect of pyruvate concentration; | |
| With sodium ruthenate(VI); hexacyanoferrate(III); sodium perchlorate; sodium hydroxide at 30℃; Kinetics; Mechanism; |

| Conditions | Yield |
|---|---|
| With acetate buffer In water at 75℃; Mechanism; Kinetics; Thermodynamic data; different buffers: pH 0.59 to 8.32; kH/kD; different temp.; Ea, ΔH(excit.), ΔS(excit.); |

| Conditions | Yield |
|---|---|
| With N-acetylneuraminic acid aldolase at 37℃; |

| Conditions | Yield |
|---|---|
| With N-acetylneuraminic acid aldolase at 37℃; |

-
A
-
72-87-7, 1136-42-1, 1136-44-3, 6082-29-7, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4
N-acetyl-β-D-mannosamine

-
B
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With N-acetylneuraminic acid aldolase at 37℃; |

| Conditions | Yield |
|---|---|
| Stage #1: L-alanin; pyridoxal hydrochloride With sodium acetate; acetic acid In ethanol; water at 50℃; pH=7.15; Stage #2: In ethanol |

-
-
1138160-36-7
sodium 4-phenyl-4-hydroxy-2-oxobutyrate

-
A
-
113-24-6
sodium pyruvate

-
B
-
100-52-7
benzaldehyde

| Conditions | Yield |
|---|---|
| With bovine heart L-lactate dehydrogenase; C80H134N14O12; NADH at 30℃; for 14h; pH=8; Kinetics; pH-value; Temperature; Reagent/catalyst; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With pyruvate aldolase BphI; manganese(ll) chloride pH=8; aq. buffer; |


-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With L-lactate dehydrogenase; NADH; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid pH=8; Kinetics; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With L-lactate dehydrogenase; NADH; manganese(ll) chloride; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid pH=8; Kinetics; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With L-lactate dehydrogenase; NADH; manganese(ll) chloride; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid pH=8; Kinetics; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With L-lactate dehydrogenase; NADH; manganese(ll) chloride; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid pH=8; Kinetics; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With L-lactate dehydrogenase; NADH; manganese(ll) chloride; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid at 25℃; pH=8; Kinetics; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With L-lactate dehydrogenase; NADH; manganese(ll) chloride; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid at 25℃; pH=8; Kinetics; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With L-lactate dehydrogenase; NADH; manganese(ll) chloride; N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid pH=8; Kinetics; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With recombinant D-lactate dehydrogenase from Lactobacillus jensenii 269-3; nicotinamide adenine dinucleotide In aq. buffer at 25℃; pH=8; Kinetics; Concentration; Reagent/catalyst; Temperature; pH-value; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With Aerococcus viridans L-lactate oxidase, wild-type, containing oxidized-state FMN cofactor In aq. phosphate buffer at 20℃; pH=6.5; Kinetics; Reagent/catalyst; Temperature; Flow reactor; |
-
-
530-26-7
D-Mannose

-
-
113-24-6
sodium pyruvate

-
-
120104-31-6
(2S,4S,5R,6R)-2,4,5-Trihydroxy-6-((1R,2R)-1,2,3-trihydroxy-propyl)-tetrahydro-pyran-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With Neu5Ac aldolase for 24h; | 100% |
| With sodium azide; potassium phosphate buffer; diothiothreitol at 37℃; for 24h; N-acetylneuraminate pyruvate lyase; | 84% |
| With sodium azide; diothiothreitol; acylneuraminate pyruvate lyase covalently bound to 4percent agarose In water at 37℃; for 24h; 0.05 M potassium phosphate buffer, pH 7.2; | 84% |
| With DL-dithiothreitol at 37℃; for 72h; NeuAc aldolase, potassium phosphate buffer, pH 7.5; | 78% |
| With sodium azide; potassium phosphate buffer; acylneuraminate pyruvate lyase; agarose; diothiothreitol at 37℃; for 24h; Yield given; |

| Conditions | Yield |
|---|---|
| With sodium formate; ammonium chloride; NADH In aq. phosphate buffer at 25℃; for 6h; pH=8.0; Kinetics; Reagent/catalyst; Green chemistry; Enzymatic reaction; | 100% |
| With zinc(II) perchlorate; (R)-15-amino-methyl-14-hydroxy-5,5-dimethyl-2,8-dithia<9>(2,5)pyridinophane In methanol for 24h; Ambient temperature; | 72% |
-
-
113-24-6
sodium pyruvate

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
139005-22-4
(E)-4-(4-bromophenyl)-2-oxo-but-3-enoic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In ethanol at 20℃; for 1h; Knoevenagel Condensation; Inert atmosphere; | 100% |
| With potassium hydroxide In ethanol | 75% |
| Stage #1: sodium pyruvate; 4-bromo-benzaldehyde In methanol; water at 0℃; for 0.166667h; Inert atmosphere; Stage #2: With potassium hydroxide In methanol; water at 0 - 20℃; Inert atmosphere; Stage #3: With hydrogenchloride In methanol; water pH=2; Inert atmosphere; | |
| With potassium hydroxide In ethanol; water at 0℃; for 3h; |
-
-
72-87-7, 1136-42-1, 1136-44-3, 6082-29-7, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4
N-acetyl-D-mannosamine

-
-
113-24-6
sodium pyruvate

-
-
229954-81-8
3-azidopropyl (β-O-D-galactopyranosyl)-(1→4)-O-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With Neisseria meningitidis CMP-sialic acid synthetase; Escherichia coli K12 sialic acid aldolase; Pasteurella multocida sialyl transferase; cytidine triphosphate; magnesium chloride pH=8.5; aq. Tris-HCl buffer; Enzymatic reaction; | 100% |


-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With Pasteurella multocida sialic acid aldolase In water at 37℃; for 48h; Enzymatic reaction; chemoselective reaction; | 100% |
| With sodium azide; sialic acid aldolase from Escherichia coli K1 In aq. phosphate buffer at 37℃; pH=7.6; Enzymatic reaction; | 63% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 25℃; | 100% |
| With [pentamethylcyclopentadienyl*Ir(N-phenyl-2-pyridinecarboxamidate)Cl]; sodium formate In methanol at 37℃; for 15h; | 100 %Spectr. |
| With cannabichromene; NADH; lactate dehydrogenase In aq. phosphate buffer Kinetics; Enzymatic reaction; | |
| With tetrahydrocannabinolic acid; NADH; lactate dehydrogenase In aq. phosphate buffer Kinetics; Enzymatic reaction; |
-
-
113-24-6
sodium pyruvate

-
-
229954-81-8
3-azidopropyl (β-O-D-galactopyranosyl)-(1→4)-O-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With E. coli sialic acid aldolase; N. meningitidis CMP-sialic acid synthetase; Photobacterium damsela α-2,6-sialyltransferase; cytidine triphosphate; magnesium chloride In water pH=8.8; | 99% |


-
-
113-24-6
sodium pyruvate

-
-
229954-81-8
3-azidopropyl (β-O-D-galactopyranosyl)-(1→4)-O-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With E. coli sialic acid aldolase; N. meningitidis CMP-sialic acid synthetase; Photobacterium damsela α-2,6-sialyltransferase; cytidine triphosphate; magnesium chloride In water pH=8.8; | 99% |

-
-
113-24-6
sodium pyruvate


| Conditions | Yield |
|---|---|
| Stage #1: sodium pyruvate; 6-azido-6-deoxy-D-mannopyranose With N. meningitidis ST3Gal-CMPNeu5Ac synthetase fusion protein; Neu5Ac-aldolase (Nal-311 (Toyobo); cytidine triphosphate In sodium hydroxide at 37℃; for 14h; pH=8.3 - 9.0; Enzymatic reaction; Stage #2: methyl β-D-galactopyranosyl-(1->4)-O-2-acetamido-2-deoxy-β-D-glucopyranoside acceptor substrate With hydrogenchloride; recombinant sialyltransferase rat ST3Gal III; manganese(ll) chloride for 14h; pH=7.0; Enzymatic reaction; Further stages.; | 98% |

-
-
72-87-7, 1136-42-1, 1136-44-3, 6082-29-7, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4
N-acetyl-D-mannosamine

-
-
113-24-6
sodium pyruvate

-
-
229954-81-8
3-azidopropyl (β-O-D-galactopyranosyl)-(1→4)-O-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With E. coli sialic acid aldolase; N. meningitidis CMP-sialic acid synthetase; Photobacterium damsela α-2,6-sialyltransferase; cytidine triphosphate; magnesium chloride In water pH=8.8; | 98% |


| Conditions | Yield |
|---|---|
| With thiamine diphosphate In phosphate buffer at 30℃; pH=6.0; | 98% |
-
-
113-24-6
sodium pyruvate

-
-
97604-58-5
2-azido-2-deoxy-D-mannopyranose

-
-
857642-16-1
(2S,3R,4S,5R,6R)-2-{[(2R,3S,4R,5R,6R)-4,5-dihydroxy-2-(hydroxymethyl)-6-(prop-2-yn-1-yloxy)tetrahydro-2H-pyran-3-yl]oxy}-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol

-
-
1196892-67-7
propargyl O-(5-azido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2->6)-O-β-D-galactopyranosyl-(1->4)-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With E. coli sialic acid aldolase; Photobacterium damsela α2,6-sialyltransferase; Neisseria meninigitidis CMP-sialic acid synthetase; cytidine-5’-triphosphate sodium salt; magnesium chloride at 20℃; for 15h; pH=8.5; aq. buffer; Enzymatic reaction; | 98% |

-
-
113-24-6
sodium pyruvate

-
-
21732-98-9
1H-[1,2,4]triazole-3-carboxylic acid hydrazide

-
-
1353017-20-5
C6H6N5O3(1-)*Na(1+)

| Conditions | Yield |
|---|---|
| In methanol for 24h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With Neisseria meningitidis CMP-sialic acid synthetase; cytidine 5′-triphosphate disodium salt; Pasteurella multocida sialic acid aldolase; Photobacterium damselae α2-6-sialyltransferase; magnesium chloride pH=8.5; aq. buffer; Enzymatic reaction; | 98% |


| Conditions | Yield |
|---|---|
| With Neisseria meningitidis CMP-sialic acid synthetase; cytidine 5′-triphosphate disodium salt; Pasteurella multocida sialic acid aldolase; Pasteurella multocida multifunctional α2-3-sialyltransferase; magnesium chloride pH=8.5; aq. buffer; Enzymatic reaction; | 98% |

-
-
113-24-6
sodium pyruvate

-
-
74950-73-5, 74950-79-1, 77870-49-6
2-acetamido-2,6-dideoxy-6-fluoro-D-mannopyranose

-
-
3150-24-1
4-nitrophenyl-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With Neisseria meningitidis CMP-sialic acid synthetase; cytidine 5′-triphosphate disodium salt; Pasteurella multocida sialic acid aldolase; Pasteurella multocida multifunctional α2-3-sialyltransferase; magnesium chloride pH=8.5; aq. buffer; Enzymatic reaction; | 98% |


| Conditions | Yield |
|---|---|
| With cytidine 5′-triphosphate disodium salt; neisseria meningitides CMP-sialicacid synthetase; pasteurella multocida sialic acidaldolase; magnesium chloride In aq. buffer at 37℃; Inert atmosphere; Enzymatic reaction; | A 98% B 92% |


| Conditions | Yield |
|---|---|
| With cytidine 5′-triphosphate disodium salt; neisseria meningitides CMP-sialicacid synthetase; pasteurella multocida sialic acidaldolase; magnesium chloride In aq. buffer at 37℃; Inert atmosphere; Enzymatic reaction; | A 98% B 95% |


| Conditions | Yield |
|---|---|
| With cytidine 5′-triphosphate disodium salt; neisseria meningitides CMP-sialicacid synthetase; pasteurella multocida sialic acidaldolase; magnesium chloride In aq. buffer at 37℃; Inert atmosphere; Enzymatic reaction; | A 98% B 80% |


| Conditions | Yield |
|---|---|
| With cytidine 5′-triphosphate disodium salt; neisseria meningitides CMP-sialicacid synthetase; pasteurella multocida sialic acidaldolase; magnesium chloride In aq. buffer at 37℃; Inert atmosphere; Enzymatic reaction; | A 98% B 89% |

-
-
19949-68-9, 37077-16-0, 60135-21-9
2,6-diacetamido-2,6-dideoxy-D-mannopyranose

-
-
1257307-68-8
3-azidopropyl β-D-galactopyranosyl-(1->3)-2-acetamido-2-deoxy-α-D-galactopyranoside

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With CTP(3Na+); Neisseria meningitidis CMP-sialic acid synthetase; pasteurella multocida sialic acidaldolase; Photobacterium damselae α2-6 sialyltransferase; magnesium chloride In aq. buffer at 37℃; pH=7.5; Enzymatic reaction; | 98% |

-
-
4419-94-7
4-nitrophenyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside

-
-
7512-17-6, 72-87-7, 1136-42-1, 1136-44-3, 6082-29-7, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4
N-Acetyl-D-glucosamine

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| Stage #1: N-Acetyl-D-glucosamine; sodium pyruvate With Escherichia coli. ΔnanTEK/pNA; magnesium chloride In aq. buffer at 30℃; for 16h; Enzymatic reaction; Stage #2: 4-nitrophenyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside With cytidine 5’-monophosphate-Sia synthetase; sialyltransferase Pm2,3ST; cytidine triphosphate at 25℃; for 2h; | 98% |

-
-
4419-94-7
4-nitrophenyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside

-
-
7512-17-6, 72-87-7, 1136-42-1, 1136-44-3, 6082-29-7, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4
N-Acetyl-D-glucosamine

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| Stage #1: N-Acetyl-D-glucosamine; sodium pyruvate With Escherichia coli. ΔnanTEK/pNA; magnesium chloride In aq. buffer at 30℃; for 16h; Enzymatic reaction; Stage #2: 4-nitrophenyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside With cytidine 5’-monophosphate-Sia synthetase; sialyltransferase Pd2,6ST; cytidine triphosphate at 25℃; for 2h; | 98% |

-
-
1152-39-2, 28244-98-6, 121523-95-3, 131488-68-1
p-methylphenyl-1-thio-β-D-galactopyranoside

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With Neisseria meningitidis CMP-sialic acid synthetase; Pasteurella multocida sialic acid aldolase; Pasteurella multocida sialyltransferase 1 M144D mutant; cytidine triphosphate; magnesium chloride In water at 30℃; for 48h; Reagent/catalyst; Enzymatic reaction; | 98% |

-
-
1152-39-2, 28244-98-6, 121523-95-3, 131488-68-1
p-methylphenyl-1-thio-β-D-galactopyranoside

-
-
113-24-6
sodium pyruvate

| Conditions | Yield |
|---|---|
| With Neisseria meningitidis CMP-sialic acid synthetase; Pasteurella multocida sialic acid aldolase; pasteurella multocida 2-3-sialyltransferase 1 M144D mutant; cytidine triphosphate; magnesium chloride In water at 30℃; for 48h; pH=8.5; Enzymatic reaction; | 98% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 0 - 25℃; | 98% |
-
-
530-26-7
D-Mannose

-
-
113-24-6
sodium pyruvate

-
-
229954-81-8
3-azidopropyl (β-O-D-galactopyranosyl)-(1→4)-O-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With E. coli sialic acid aldolase; N. meningitidis CMP-sialic acid synthetase; Photobacterium damsela α-2,6-sialyltransferase; cytidine triphosphate; magnesium chloride In water pH=8.8; | 97% |


| Conditions | Yield |
|---|---|
| With Neisseria meningitidis CMP-sialic acid synthetase; Pasturella multocida sialic acid aldolase; Photobacterium damsela α2-6-sialyltransferase; magnesium chloride; cytidine 5'-triphosphate disodium salt In water at 37℃; pH=8.8; aq. Tris-HCl buffer; Enzymatic reaction; | 97% |

-
-
113-24-6
sodium pyruvate

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
250641-14-6
6-bromoquinoline-2 ,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 4h; Reflux; Inert atmosphere; | 97% |
| With water; sodium hydroxide at 110℃; under 4500.45 Torr; for 0.166667h; Microwave irradiation; | 92% |
| With sodium hydroxide In water at 110℃; under 3750.38 Torr; for 0.333333h; Microwave irradiation; | 81.1% |
Sodium Pyruvate Chemical Properties
IUPAC Name: Sodium 2-oxopropanoate
Synonyms of of Sodium Pyruvate (CAS NO.113-24-6): 2-Oxopropanoate de sodium ; Natrium-2-oxopropanoat ; Propanoic acid, 2-oxo-, sodium salt ; Propanoic acid, 2-oxo-, sodium salt (1:1) ; Sodium 2-oxopropanoate
Molecular Structure: 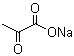
Molecular Formula: C3H3NaO3
Molecular Weight: 110.04
CAS NO : 113-24-6
EINECS : 204-024-4
BRN : 3568341
H bond acceptors: 3
H bond donors: 1
Freely Rotating Bonds: 1
Polar Surface Area: 54.37 Å2
Flash Point: 54.3 °C
Enthalpy of Vaporization: 44.28 kJ/mol
Boiling Point: 165 °C at 760 mmHg
Vapour Pressure: 0.968 mmHg at 25°C
Melting Point: >300 °C(lit.)
Density: 1.267g/cm3
Refractive index: 1.426-1.43
Storage temp: 2-8°C
Solubility: H2O: 100 mg/mL
Stability: Stable. Incompatible with strong oxidizing agents.
Appearance:White to slightly yellow crystalline powder
Product Categories of Sodium Pyruvate (CAS NO.113-24-6): FINE Chemical & INTERMEDIATES;PyruvicAcidSeries;Hybridoma Media SupplementsCell Culture;Hybridoma Platform;Miscellaneous Reagents and SupplementsNeural Stem Cell Biology;Neural Stem Cell Isolation/Expansion;Reagents and Supplements;Serum-free Media;Supplements/Reagents
Sodium Pyruvate Uses
Sodium Pyruvate (CAS NO.113-24-6) is commonly added to cell culture media as an additional source of energy, but may also have protective effects against hydrogen peroxide. This was reported by Giandomenico et al. and has been confirmed by several independent groups.
Sodium Pyruvate Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 36/37/39-26-24/25
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S24/25: Avoid contact with skin and eyes.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 3
HS Code: 29183000
Related Products
- Sodium
- Sodium 2,4-dimethylbenzenesulfonate
- Sodium ((3-methoxy-1-methyl-3-oxo-1-propenyl)amino)phenylacetate
- Sodium (+)-10-camphorsulfonate
- Sodium (2-carbamoylphenoxy)acetate
- Sodium (2-methyl-4-chlorophenoxy)acetate
- Sodium (6R,7R)-3-[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanylmethyl]-8-oxo-7-[[2-(tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate pentahydrate
- Sodium (C10-16)alkylbenzenesulfonate
- Sodium (R,R)-5-(2-((2-(3-chlorophenyl)-2-hydroxyethyl)amino)propyl)-1,3-benzodioxole-2,2-
- Sodium 1,3-benzothiazole-2-thiolate
- 113246-86-9
- 113248-64-9
- 1132610-98-0
- 1132-61-2
- 1132672-11-7
- 1132-68-9
- 113269-09-3
- 113270-98-7
- 113270-99-8
- 113274-56-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View