-
Name
Terpinen-4-ol
- EINECS 209-235-5
- CAS No. 562-74-3
- Article Data30
- CAS DataBase
- Density 0.933 g/cm3
- Solubility Slightly soluble in water, soluble in alcohol and oil
- Melting Point 137-188 °C
- Formula C10H18O
- Boiling Point 208.999 °C at 760 mmHg
- Molecular Weight 154.252
- Flash Point 79.444 °C
- Transport Information
- Appearance colourless or pale yellow liquid
- Safety 26-36-37/39
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms p-Menth-1-en-4-ol(8CI);1-(1-Methylethyl)-4-methyl-3-cyclohexen-1-ol;1-Isopropyl-4-methylcyclohex-3-en-1-ol;1-Terpinen-4-ol;4-Carvomenthenol;4-Methyl-1-(1-methylethyl)-3-cyclohexen-1-ol;4-Methyl-1-(1-methylethyl)-3-cyclohexene-1-ol;4-Methyl-1-isopropyl-3-cyclohexen-1-ol;4-Terpinenol;4-Terpineol;Melaleucol;NSC 147749;Terpin-4-ol;Terpinene-4-ol;Terpineol-4;dl-4-Terpineol;
- PSA 20.23000
- LogP 2.50370
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In water; ethyl acetate at 50℃; under 75.0075 Torr; for 5h; Solvent; Concentration; Temperature; Large scale; | 97.8% |
-
-
125816-48-0
(1-Isopropyl-4-methyl-cyclohex-3-enyloxymethoxy)-dimethyl-(1,1,2-trimethyl-propyl)-silane

-
-
562-74-3
TERPINEN-4-OL

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 2.5h; Product distribution; Ambient temperature; cleavage with Et4NF in MeCN; cleavage of acetals with tetraalkylammonium fluoride salts; protection and deprotection of alcohols with chloromethoxysilanes; | 78% |
-
-
4584-23-0
2,2,6-trimethyl-1-oxa-spira[2.5]oct-ene

-
A
-
562-74-3
TERPINEN-4-OL

-
B
-
3419-02-1
4-methyl-1-isopropenylcyclohex-3-en-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In ethyl acetate at 25 - 125℃; under 3750.38 Torr; for 24h; Temperature; Reagent/catalyst; Solvent; Time; Autoclave; Inert atmosphere; Overall yield = 82.7 %; | A 8.3% B 74.4% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; aluminium trichloride In diethyl ether for 8h; Ambient temperature; | A 67% B 22% |
| With lithium In ethylenediamine for 0.5h; | A 36% B 60% |
-
-
105273-31-2
Acetic acid (Z)-3,7-dimethyl-6-oxo-oct-2-enyl ester

-
-
562-74-3
TERPINEN-4-OL

| Conditions | Yield |
|---|---|
| With samarium diiodide; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 3h; Heating; | 62% |

| Conditions | Yield |
|---|---|
| With Penicillium paxilli In dimethyl sulfoxide at 28 - 30℃; for 120h; Reagent/catalyst; Microbiological reaction; | A 56% B 3% C 13% |
| With Fusarium concentricum In dimethyl sulfoxide at 28 - 30℃; for 120h; Reagent/catalyst; Microbiological reaction; | A 30% B 21% C 17% |


| Conditions | Yield |
|---|---|
| With n-butyllithium; potassium tert-butylate In hexane at 60℃; for 40h; | 50% |
-
-
4584-23-0
2,2,6-trimethyl-1-oxa-spira[2.5]oct-ene

-
A
-
586-62-9
Terpinolene

-
B
-
1197-01-9
p-cymene-8-ol

-
C
-
562-74-3
TERPINEN-4-OL

-
D
-
98-55-5
terpineol

| Conditions | Yield |
|---|---|
| With sodium In tetrahydrofuran at 65℃; for 12h; | A 4% B 16% C 48% D 26% |

-
-
2009-00-9, 3387-41-5, 10408-16-9, 15826-80-9
(±)-sabinene

-
-
562-74-3
TERPINEN-4-OL

| Conditions | Yield |
|---|---|
| With formic acid Verseifen des Formiats; dextrorotatory terpinenol-(4); |
-
-
2009-00-9, 3387-41-5, 10408-16-9, 15826-80-9
(±)-sabinene

-
A
-
562-74-3
TERPINEN-4-OL

-
B
-
91008-90-1
terpin-4-ol

| Conditions | Yield |
|---|---|
| With sulfuric acid dextrorotatory terpinenol-(4); |
-
-
4584-23-0
2,2,6-trimethyl-1-oxa-spira[2.5]oct-ene

-
A
-
1195-32-0
1-methyl-4-isopropenylbenzene

-
B
-
1197-01-9
p-cymene-8-ol

-
C
-
99-87-6
4-methylisopropylbenzene

-
D
-
562-74-3
TERPINEN-4-OL

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 66℃; for 7.5h; Further byproducts given. Title compound not separated from byproducts; |

-
-
4584-23-0
2,2,6-trimethyl-1-oxa-spira[2.5]oct-ene

-
A
-
1195-32-0
1-methyl-4-isopropenylbenzene

-
B
-
1197-01-9
p-cymene-8-ol

-
C
-
99-87-6
4-methylisopropylbenzene

-
D
-
562-74-3
TERPINEN-4-OL

-
E
-
53312-55-3
p-mentha-1,4-dien-8-ol

-
F
-
82538-84-9
1-(1'-hydroxy-1'-methylethyl)-4-methylcyclohexa-1,3-diene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 27℃; for 0.75h; Product distribution; var. solvents and temp.; |


| Conditions | Yield |
|---|---|
| at 100℃; trans-form; |


| Conditions | Yield |
|---|---|
| levorotatory form; |

-
-
562-74-3
TERPINEN-4-OL

| Conditions | Yield |
|---|---|
| With sulfuric acid dextrorotatory terpinenol-(4); |

| Conditions | Yield |
|---|---|
| With oxalic acid inactive terpinenol-(4); |

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 50 - 100℃; inactive terpinenol-(4); |

| Conditions | Yield |
|---|---|
| With anthracene; sodium In water |
-
-
763-10-0
geranyl diphosphate

-
A
-
106-24-1
Geraniol

-
B
-
562-74-3
TERPINEN-4-OL

-
C
-
471-84-1, 2623-54-3, 7378-37-2, 116724-26-6
fenchene

| Conditions | Yield |
|---|---|
| With 6His-tagged Pseudomonas fluorescens PfO-1 Pfl_1841 2-methylenebornane synthase In pentane at 30℃; for 12h; pH=6.7; Kinetics; Concentration; aq. buffer; Enzymatic reaction; | A 6 %Chromat. B 5 %Chromat. C 52 %Chromat. |

-
-
562-74-3
TERPINEN-4-OL

-
-
18663-30-4
Benz-phenylimidsaeure-triaethylsilylester

| Conditions | Yield |
|---|---|
| With pyridinium triflate In tetrahydrofuran at 25℃; for 0.166667h; | 99% |
-
-
562-74-3
TERPINEN-4-OL

-
-
404392-70-7
tert-butyldimethylsilyl N-phenylbenzimidate

| Conditions | Yield |
|---|---|
| With pyridinium triflate In tetrahydrofuran at 50℃; for 2.5h; | 96% |
-
-
562-74-3
TERPINEN-4-OL

-
-
96622-71-8
C16H19NOSi

| Conditions | Yield |
|---|---|
| With pyridinium triflate In tetrahydrofuran at 25℃; for 0.0833333h; | 95% |
-
-
562-74-3
TERPINEN-4-OL

-
-
53742-62-4
anilino(tert-butyldimethyl)silane

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In N,N-dimethyl-formamide at 20℃; for 3h; | 92% |

| Conditions | Yield |
|---|---|
| With N.N'-bis[3,5-bis(trifluoromethyl)phenyl]thiourea at 50℃; for 51h; | 92% |
-
-
562-74-3
TERPINEN-4-OL

-
-
18106-48-4
1,1,1-triethyl-N-phenylsilanamine

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In N,N-dimethyl-formamide at 20℃; for 0.5h; | 91% |

| Conditions | Yield |
|---|---|
| With C12H8N2*2CH4O3S at 40℃; for 5h; | 89% |
| With sodium acetate In xylene | |
| With pyridine In dichloromethane Acetylation; |

| Conditions | Yield |
|---|---|
| Stage #1: TERPINEN-4-OL With tert-butylmagnesium chloride In tetrahydrofuran; diethyl ether Cooling; Inert atmosphere; Stage #2: 1,2-dibromomethane With tert-butylmagnesium chloride In tetrahydrofuran; diethyl ether at 20℃; for 2h; Inert atmosphere; optical yield given as %de; | 89% |
| With tert-butylmagnesium chloride In diethyl ether at 25℃; for 16h; | 85% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 20℃; for 18h; Inert atmosphere; | 82% |
| With potassium phosphate; copper(I) bromide dimethylsulfide complex; tricyclohexylphosphine In acetonitrile at 80℃; for 24h; | 48% |
-
-
562-74-3
TERPINEN-4-OL

-
-
125816-34-4
1-chloro-3,3,4,4,5-pentamethyl-2-oxa-3-silahexane

-
-
125816-48-0
(1-Isopropyl-4-methyl-cyclohex-3-enyloxymethoxy)-dimethyl-(1,1,2-trimethyl-propyl)-silane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 45h; Ambient temperature; | 80% |
-
-
562-74-3
TERPINEN-4-OL

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; dicyclohexyl-carbodiimide In water Ambient temperature; other reagent: H2O2/KHCO3/benzonitrile; | 80% |

| Conditions | Yield |
|---|---|
| With iron(III)-acetylacetonate; methyl 4-nitrobenzenesulfonate; phenylsilane; sodium hydrogencarbonate In methanol at 0 - 20℃; for 12h; Schlenk technique; Inert atmosphere; diastereoselective reaction; | 76% |
-
-
562-74-3
TERPINEN-4-OL

-
-
22621-68-7
2-hydroxy-1,4-cineole

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In chloroform at 25℃; for 0.5h; Concentration; Solvent; Temperature; | 70.1% |
-
-
562-74-3
TERPINEN-4-OL

-
-
74-88-4
methyl iodide

-
-
94281-60-4
4-isopropyl-4-methoxy-1-methylcyclohex-1-ene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In diethyl ether at 20℃; for 72h; Schlenk technique; | 60% |
-
-
562-74-3
TERPINEN-4-OL

-
-
2873-29-2
D-glucal triacetate

-
-
1619941-96-6
α-terpinyl 4,6-di-O-acetyl-2,3-dideoxy-α-D-erythro-hex-2-enopyranoside

| Conditions | Yield |
|---|---|
| With zinc dibromide In chloroform at 72℃; for 0.916667h; Ferrier Carbohydrate Rearrangement; Microwave irradiation; stereoselective reaction; | 56% |
-
-
562-74-3
TERPINEN-4-OL

-
-
616-38-6
carbonic acid dimethyl ester

-
-
1262126-86-2
methyl 4-terpinyl carbonate

| Conditions | Yield |
|---|---|
| Stage #1: carbonic acid dimethyl ester With lanthanum(III) isopropoxide; 2-(2-methoxyethoxy)ethyl alcohol at 20℃; Stage #2: TERPINEN-4-OL for 89h; Reflux; chemoselective reaction; | 55% |
-
-
562-74-3
TERPINEN-4-OL

-
B
-
98109-59-2
para-menthane-1,2,4-triol

| Conditions | Yield |
|---|---|
| With 3-Methylpyrazole; dihydrogen peroxide; methyltrioxorhenium(VII) at 10 - 20℃; for 2h; | A n/a B 42% |
-
-
562-74-3
TERPINEN-4-OL

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
99049-84-0, 99049-85-1
1-p-Menthen-2,3,4,6-tetra-O-acetyl-β-D-glucopyranosid

| Conditions | Yield |
|---|---|
| With molecular sieve; silver silicate In dichloromethane for 48h; Ambient temperature; | 21% |
-
-
562-74-3
TERPINEN-4-OL

-
-
25570-96-1
1,4-dibromo-p-menthane

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid trans-form; |
-
-
562-74-3
TERPINEN-4-OL

-
-
27621-71-2
1,4-dichloro-p-menthane

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid trans-form; |
-
-
562-74-3
TERPINEN-4-OL

-
-
25570-95-0
1,4-dichloro-trans-p-menthane

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid |

| Conditions | Yield |
|---|---|
| With nickel at 125 - 130℃; under 5884.06 Torr; Hydrogenation; | |
| With (bis-1,2-diphenylphosphinoethane)Co(CH2SiMe3)2; hydrogen In toluene at -196.15 - 25℃; under 3040.2 Torr; for 4h; Reagent/catalyst; Sealed tube; diastereoselective reaction; | n/a |
| With C54H57F3N4O6RuS; hydrogen In dichloromethane at 80℃; under 37503.8 Torr; for 13h; Reagent/catalyst; Inert atmosphere; Sealed tube; | |
| With hydrogen In toluene at 80℃; under 2250.23 Torr; Catalytic behavior; Activation energy; Reagent/catalyst; Temperature; Autoclave; diastereoselective reaction; | n/a |
Terpinen-4-ol Consensus Reports
Terpinen-4-ol Specification
The Terpinen-4-ol, with the CAS registry number 562-74-3, is also known as 4-Methyl-1-(1-methylethyl)-3-cyclohexen-1-ol. It belongs to the product categories of Biochemistry; Terpenes; Terpenes (Others); Monocyclic Monoterpenes; Intermediates & Fine Chemicals; Pharmaceuticals; Alphabetical Listings; C-D Flavors and Fragrances; Certified Natural Products; Flavors and Fragrances. Its EINECS number is 209-235-5. This chemical's molecular formula is C10H18O and molecular weight is 154.25. What's more, its systematic name is 1-Isopropyl-4-methyl-3-cyclohexen-1-ol. Its classification codes are: (1)Drug / Therapeutic Agent; (2)Natural Product; (3)Skin / Eye Irritant. This chemical is mainly the primary active ingredient of tea tree oil. It is also the compound of highest concentration in the essential oil of nutmeg. It shows antioxidant effects and is an antiseptic.
Physical properties of Terpinen-4-ol are: (1)ACD/LogP: 2.538; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.54; (4)ACD/LogD (pH 7.4): 2.54; (5)ACD/BCF (pH 5.5): 50.01; (6)ACD/BCF (pH 7.4): 50.01; (7)ACD/KOC (pH 5.5): 572.50; (8)ACD/KOC (pH 7.4): 572.50; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.485; (14)Molar Refractivity: 47.339 cm3; (15)Molar Volume: 165.26 cm3; (16)Polarizability: 18.766×10-24cm3; (17)Surface Tension: 33 dyne/cm; (18)Density: 0.933 g/cm3; (19)Flash Point: 79.444 °C; (20)Enthalpy of Vaporization: 51.794 kJ/mol; (21)Boiling Point: 208.999 °C at 760 mmHg; (22)Vapour Pressure: 0.048 mmHg at 25°C.
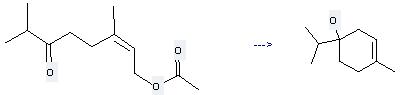
Preparation: this chemical can be prepared by acetic acid 3,7-dimethyl-6-oxo-oct-2-enyl ester by heating. This reaction will need reagent SmI2 and solvent tetrahydrofuran with the reaction time of 3 hours. This reaction will also need catalyst Pd(PPh3)4. The yield is about 62%.
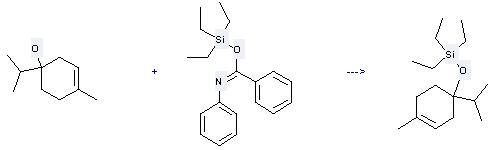
Uses of Terpinen-4-ol: it can be used to produce triethyl-(1-isopropyl-4-methyl-cyclohex-3-enyloxy)-silane at the temperature of 25 °C. It will need reagent PyH(+)·OTf(-) and solvent tetrahydrofuran with the reaction time of 10 min. The yield is about 99%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. It is harmful if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: CC1=CCC(CC1)(C(C)C)O
(2)Std. InChI: InChI=1S/C10H18O/c1-8(2)10(11)6-4-9(3)5-7-10/h4,8,11H,5-7H2,1-3H3
(3)Std. InChIKey: WRYLYDPHFGVWKC-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intramuscular | 780mg/kg (780mg/kg) | KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED | Cesko-Slovenska Farmacie. Vol. 8, Pg. 433, 1959. |
| mouse | LD50 | intraperitoneal | 250mg/kg (250mg/kg) | KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED | Cesko-Slovenska Farmacie. Vol. 8, Pg. 433, 1959. |
| mouse | LD50 | oral | 1016mg/kg (1016mg/kg) | Zhonghua Jiehe He Huxixi Jibing Zazhi. Chinese Journal of Tuberculosis and Respiratory Diseases. Vol. 4(4), Pg. 203, 1981. | |
| mouse | LD50 | subcutaneous | 750mg/kg (750mg/kg) | KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED | Cesko-Slovenska Farmacie. Vol. 8, Pg. 433, 1959. |
| rabbit | LD50 | skin | > 2500mg/kg (2500mg/kg) | Food and Chemical Toxicology. Vol. 20, Pg. 833, 1982. | |
| rat | LD50 | intramuscular | 1500mg/kg (1500mg/kg) | KIDNEY, URETER, AND BLADDER: URINE VOLUME INCREASED | Cesko-Slovenska Farmacie. Vol. 8, Pg. 433, 1959. |
| rat | LD50 | oral | 1300mg/kg (1300mg/kg) | Food and Chemical Toxicology. Vol. 20, Pg. 833, 1982. |
Related Products
- Terpinen-4-ol
- 56275-95-7
- 56278-50-3
- 562803-76-3
- 56281-37-9
- 562824-27-5
- 562825-95-0
- 5628-44-4
- 562858-09-7
- 56287-72-0
- 56287-73-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View