-
Name
Tetrabutylphosphonium bromide
- EINECS 221-487-8
- CAS No. 3115-68-2
- Article Data11
- CAS DataBase
- Density 1.8[at 20℃]
- Solubility 70 g/100 mL in water
- Melting Point 99-104 ºC
- Formula C16H36BrP
- Boiling Point
- Molecular Weight 339.34
- Flash Point 290 ºC
- Transport Information UN 3464
- Appearance white to cream crystalline powder
- Safety 26-37/39-45-36/37/39-28A-36
- Risk Codes 21/22-36/37/38-24-20/21/22
-
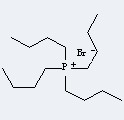
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi,
Xi, T
T
- Synonyms Phosphonium,tetrabutyl-, bromide (8CI,9CI);Tetrabutylphosphonium bromide (6CI,7CI);Cyphos443W;NSC 41942;PX 4B;T 1124;TBP-BB;Tetra-n-butylphosphonium bromide;
- PSA 13.59000
- LogP 3.20840
Synthetic route

| Conditions | Yield |
|---|---|
| With samarium(III) chloride; phosphorous In acetonitrile at 50℃; electrosynthesis; | 10% |

| Conditions | Yield |
|---|---|
| With P |
-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| With potassium hydrogen bifluoride In dichloromethane for 0.5h; Ambient temperature; | 100% |
-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| With triethyloxonium fluoroborate In dichloromethane at 20℃; for 0.5h; | 100% |
| With trimethyl phosphite; tetrafluoroboric acid at 0 - 60℃; for 15h; Inert atmosphere; neat (no solvent); | 94% |
| With sodium tetrafluoroborate In water | 93% |
| With sodium tetrafluoroborate In dichloromethane at 120℃; for 24h; Inert atmosphere; Schlenk technique; Glovebox; | 90% |
-
-
31299-31-7, 77519-93-8
cis-dibromobis(trimethylphosphite-κP)platinum

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
1478658-04-6
tetrabutylphosphonium cis-dibromo(dimethylphosphonato)(trimethylphosphite)platinum(II)

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| In water | 99% |
-
-
13755-29-8
sodium tetrafluoroborate

-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| In pentan-1-ol | 98.6% |
-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
1885-14-9
phenyl chloroformate

-
-
616-38-6
carbonic acid dimethyl ester

-
-
102-09-0
bis(phenyl) carbonate

| Conditions | Yield |
|---|---|
| 98% |

-
-
13509-27-8
methyl phenyl carbonate

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
1885-14-9
phenyl chloroformate

-
-
102-09-0
bis(phenyl) carbonate

| Conditions | Yield |
|---|---|
| 98% |

-
-
2926-27-4
potassium trifluoromethansulfonate

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
126991-62-6
tetra-n-butylphosphonium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In water | 98% |
-
-
13509-27-8
methyl phenyl carbonate

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
98-88-4
benzoyl chloride

-
-
93-99-2
benzoic acid phenyl ester

| Conditions | Yield |
|---|---|
| 97% |


-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane for 1h; | 97% |
-
-
13509-27-8
methyl phenyl carbonate

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
2555-49-9
phenoxyacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| 96% |

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
126991-62-6
tetra-n-butylphosphonium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With trimethyl phosphite at 0 - 60℃; for 15h; Inert atmosphere; neat (no solvent); | 96% |
-
-
1492-52-0
methyl (S)-2-(benzyloxycarbonlamino)-3-(p-toluenesulfonyl)propionate

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
584-08-7
potassium carbonate

-
-
108-98-5
thiophenol

-
-
153277-33-9
N-benzyloxycarbonyl-S-phenyl-L-cysteine methyl ester

| Conditions | Yield |
|---|---|
| In water; toluene | 94.6% |

-
-
90841-17-1
2-bromo-N-(4-chlorophenyl)butanamide

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
72468-65-6
N-(4-chloro)phenyl-2-hydroxy butyramide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium formate In water; toluene | 94% |
-
-
916079-45-3
bis[bis(pentafluoroethyl)phosphinyl]imide

-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| In water at 20℃; | 94% |
| In water at 20℃; | 86% |

| Conditions | Yield |
|---|---|
| In toluene at 150℃; under 18751.9 Torr; for 15h; Autoclave; | 94% |
| In toluene pptd. (Et2O); |

-
-
10213-10-2
sodium tungstate (VI) dihydrate

-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| With HNO3 In methanol; water byproducts: H2O; (C5(CH3)5)W2O5 in methanol was mixed with an aq. soln. of Na2WO4*2H2O, aq. HNO3 was added, stirring for 2 h, bromide salt in water was added; ppt. was filtered off, washed with small portions of water, methanol anddiethyl ether, dried under vac. at 70°C; elem. anal.; | 94% |

-
-
1912-31-8
propyl methanesulfonate

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
A
-
106-94-5
propyl bromide

-
B
-
98342-59-7
tetrabutylphosphonium methanesulfonate

| Conditions | Yield |
|---|---|
| In benzene at 80℃; | A n/a B 93% |

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
124005-47-6
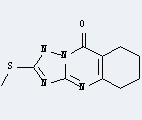
2-methylsulfanyl-6,7,8,9-tetrahydro-1,2,4-triazolo[5,1-b]quinazoline-5(10H)-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform at 20℃; for 0.0833333h; | 93% |

-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| In acetone | 93% |

-
-
109-79-5
1-butanethiol

-
-
993-43-1
ethylphosphonothioic dichloride

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
17162-58-2
S-n-butyl ethylphosphonodithioic chloride

| Conditions | Yield |
|---|---|
| With sodium carbonate In diethyl ether | 92.5% |


| Conditions | Yield |
|---|---|
| With HNO3 In methanol; water byproducts: H2O; (C5(CH3)5)Mo2O5 in methanol was mixed with an aq. soln. of Na2MoO4*2H2O,aq. HNO3 was added, stirring for 2 h, bromide salt in water was added; ppt. was filtered off, washed with small portions of water, methanol anddiethyl ether, dried under vac. at 70°C; elem. anal.; | 92% |


| Conditions | Yield |
|---|---|
| In water for 0.166667h; | 92% |

-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
916730-42-2
tetra-n-butylphosphonium tetracyanidoborate

| Conditions | Yield |
|---|---|
| In acetonitrile for 0.25h; | 91% |
| In acetonitrile byproducts: KBr; solns. of K(B(CN)4) (1.624 mmol) and P(C4H9)4Br mixed, stireed for 15 min; centrifuged, solvent evapd., ppt. washed (Et2O), dried at room temp. (under vac.), recrystd. from CH3CN; elem. anal.; | 91% |

-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| With nitric acid In water for 0.0833333h; pH=4.5; | 91% |
-
-
160298-76-0
silver(I) tetrakis(3,5-bis(trifluoromethyl)phenyl)borate

-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| In dichloromethane at 25℃; for 0.166667h; Darkness; Schlenk technique; | 91% |
-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| With trimethyl phosphite; nitric acid at 0 - 60℃; for 15h; Inert atmosphere; neat (no solvent); | 90% |
-
-
3115-68-2
tetrabutyl phosphonium bromide

-
-
90076-65-6
bis(trifluoromethane)sulfonimide lithium

| Conditions | Yield |
|---|---|
| In dichloromethane at 120℃; for 24h; Inert atmosphere; Schlenk technique; Glovebox; | 90% |
| In water for 2h; |

| Conditions | Yield |
|---|---|
| In dichloromethane; water at 20℃; for 1h; | 90% |

-
-
3115-68-2
tetrabutyl phosphonium bromide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In hexane; water at 20℃; for 6h; | 90% |
Tetrabutylphosphonium bromide Consensus Reports
Tetrabutylphosphonium bromide Specification
The IUPAC name of Tetrabutylphosphonium bromide is tetrabutylphosphanium bromide. With the CAS registry number 3115-68-2, it is also named as Phosphonium, tetrabutyl-, bromide. The product's categories are Phosphonium Salts; Quaternary phosphonium salts; Ammonium, Phosphonium, Sulfonium Salts (Ionic Liquids); Ionic Liquids; Phosphonium Compounds; Synthetic Organic Chemistry; Phosphonium Salts; Greener Alternatives: Catalysis; Phase Transfer Catalysts, and the other registry number is 34283-24-4. Besides, it is white to cream crystalline powder, which should be closed in a cool and dry place. In addition, it is soluble in methanol, acetone, toluene.
The other characteristics of this product can be summarized as: (1)H-Bond Donor: 0; (2)H-Bond Acceptor: 1; (3)Rotatable Bond Count: 12; (4)Exact Mass: 338.1738; (5)MonoIsotopic Mass: 338.1738; (6)Topological Polar Surface Area: 0; (7)Heavy Atom Count: 18; (8)Formal Charge: 0; (9)Complexity: 116; (10)Melting point: 99-104 °C; (11)Flash point: 290 °C; (12)Water solubility: 70 g/100 mL; (13)EINECS: 221-487-8.
Uses of Tetrabutylphosphonium bromide: it can react with 2-Methylsulfanyl-5,6,7,8-tetrahydro-4H-[1,2,4]triazolo[5,1-b]quinazolin-9-one to get Tetrabutyl-phosphonium; 2-methylsulfanyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[5,1-b]quinazolin-9-olate.
![]()
![]()

This reaction needs aq. NaOH and CHCl3 at temperature of 20 °C for 5 min. The yield is 93 %.
When you are using this chemical, please be cautious about it as the following: it is harmful by inhalation, in contact with skin and if swallowed and toxic in contact with skin. And it is also irritating to eyes, respiratory system and skin. You should wear suitable protective clothing, gloves and eye/face protection when use it. After contact with skin, wash immediately with plenty of soap-suds. Moreover, in case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
People can use the following data to convert to the molecule structure.
(1)SMILES:[Br-].CCCC[P+](CCCC)(CCCC)CCCC
(2)InChI:InChI=1/C16H36P.BrH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
(3)InChIKey:RKHXQBLJXBGEKF-REWHXWOFAQ
(4)Std. InChI:InChI=1S/C16H36P.BrH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
(5)Std. InChIKey:RKHXQBLJXBGEKF-UHFFFAOYSA-M
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 56mg/kg (56mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#03131, | |
| rabbit | LD50 | skin | 197mg/kg (197mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | National Technical Information Service. Vol. OTS0535940, |
| rat | LC50 | inhalation | > 3mg/m3/1H (3mg/m3) | Toxicology. Vol. 24, Pg. 245, 1982. | |
| rat | LD50 | oral | 420mg/kg (420mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES LIVER: OTHER CHANGES | National Technical Information Service. Vol. OTS0535942, |
Related Products
- Tetrabutylphosphonium acetate
- Tetrabutylphosphonium acetate acetic acid salt
- Tetrabutylphosphonium bromide
- Tetrabutylphosphonium chloride
- Tetrabutylphosphonium fluoride
- Tetrabutylphosphonium hydroxide
- Tetrabutylphosphonium iodide
- 31161-46-3
- 31165-67-0
- 31166-29-7
- 31166-44-6
- 3116-76-5
- 31168-10-2
- 31169-25-2
- 31169-27-4
- 3117-02-0
- 3117-03-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View