-
Name
Tetrahydropapaverine hydrochloride
- EINECS 229-213-9
- CAS No. 6429-04-5
- Article Data4
- CAS DataBase
- Density 1.12g/cm3
- Solubility Soluble in water
- Melting Point 213°C~215°C
- Formula C20H25NO4.HCl
- Boiling Point 475.8 °C at 760 mmHg
- Molecular Weight 379.884
- Flash Point 202.7 °C
- Transport Information
- Appearance White crystal powder
- Safety 36
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols

- Synonyms Isoquinoline,1,2,3,4-tetrahydro-6,7-dimethoxy-1-veratryl-, hydrochloride (6CI,7CI,8CI);(?à)-Norlaudanosine hydrochloride;(?à)-Tetrahydropapaverine hydrochloride;1,2,3,4-Tetrahydropapaverine hydrochloride;NSC 118072;NZ 54;Tetrahydropapaverine hydrochloride;
- PSA 48.95000
- LogP 4.28130
Synthetic route
-
-
5884-22-0
1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-3,4-dihydroisoquinoline hydrochloride

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate at 0 - 20℃; for 0.166667h; Inert atmosphere; | 89% |
-
-
6957-27-3
3,4-dihydropapaverine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With sodium (S)-prolinate-borane In tetrahydrofuran for 240h; Ambient temperature; | 78% |
| With sodium tetrahydroborate for 19h; Ambient temperature; | 71% |
-
-
13074-31-2
Tetrahydropapaverine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol | 66% |
-
-
93-40-3
(3,4-Dimethoxyphenyl)acetic acid

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / 2 h / 190 - 210 °C 2: phosphoryl chloride / toluene / 3 h / Heating 3: 71 percent / sodium borohydride / 19 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1: 1 h / 180 °C 2: trichlorophosphate / acetonitrile / 1 h / Reflux; Inert atmosphere 3: sodium tetrahydroborate; methanol / 0.17 h / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
139-76-4
N-(3,4-dimethoxyphenethyl)-2-(3,4-dimethoxyphenyl)acetamide

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: phosphoryl chloride / toluene / 3 h / Heating 2: 71 percent / sodium borohydride / 19 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: trichlorophosphate / acetonitrile / 1 h / Reflux; Inert atmosphere 2: sodium tetrahydroborate; methanol / 0.17 h / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / 2 h / 190 - 210 °C 2: phosphoryl chloride / toluene / 3 h / Heating 3: 71 percent / sodium borohydride / 19 h / Ambient temperature View Scheme |
-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1 h / 180 °C 2: trichlorophosphate / acetonitrile / 1 h / Reflux; Inert atmosphere 3: sodium tetrahydroborate; methanol / 0.17 h / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
16659-88-4
(+/-)-Tetrahydropapaveroline hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide for 2.5h; Reflux; | 95.1% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
109-94-4
formic acid ethyl ester

-
-
50722-30-0
1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol for 40h; Bischler-Napieralski Reaction; Reflux; | 94% |
-
-
93-40-3
(3,4-Dimethoxyphenyl)acetic acid

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 17h; | 89% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
54417-53-7
(R)-tetrahydropapaverine hydrochloride

| Conditions | Yield |
|---|---|
| With N-acetyl-D-leucine; (R)-N-acetylphenylalanin In water; toluene; acetonitrile at 4 - 70℃; for 24.92h; Reagent/catalyst; | 83.25% |
-
-
50-00-0
formaldehyd

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
523-02-4, 4216-86-8, 6872-27-1, 13407-95-9
2,3,10,11-tetramethoxy-5,6,7,8,13,13a-hexahydroisoquinolino[3,2-a]isoquinoline

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 8h; | 80% |
| With acetic acid at 100℃; for 0.5h; Pictet-Spengler Synthesis; | 73% |
| In water Cyclization; Heating; | |
| In water for 30h; Heating; | 8.2 g |
-
-
5884-22-0
1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-3,4-dihydroisoquinoline hydrochloride

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate at 0 - 20℃; for 0.166667h; Inert atmosphere; | 89% |
-
-
6957-27-3
3,4-dihydropapaverine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With sodium (S)-prolinate-borane In tetrahydrofuran for 240h; Ambient temperature; | 78% |
| With sodium tetrahydroborate for 19h; Ambient temperature; | 71% |
-
-
13074-31-2
Tetrahydropapaverine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol | 66% |
-
-
93-40-3
(3,4-Dimethoxyphenyl)acetic acid

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / 2 h / 190 - 210 °C 2: phosphoryl chloride / toluene / 3 h / Heating 3: 71 percent / sodium borohydride / 19 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1: 1 h / 180 °C 2: trichlorophosphate / acetonitrile / 1 h / Reflux; Inert atmosphere 3: sodium tetrahydroborate; methanol / 0.17 h / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
139-76-4
N-(3,4-dimethoxyphenethyl)-2-(3,4-dimethoxyphenyl)acetamide

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: phosphoryl chloride / toluene / 3 h / Heating 2: 71 percent / sodium borohydride / 19 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: trichlorophosphate / acetonitrile / 1 h / Reflux; Inert atmosphere 2: sodium tetrahydroborate; methanol / 0.17 h / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / 2 h / 190 - 210 °C 2: phosphoryl chloride / toluene / 3 h / Heating 3: 71 percent / sodium borohydride / 19 h / Ambient temperature View Scheme |
-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1 h / 180 °C 2: trichlorophosphate / acetonitrile / 1 h / Reflux; Inert atmosphere 3: sodium tetrahydroborate; methanol / 0.17 h / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
16659-88-4
(+/-)-Tetrahydropapaveroline hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide for 2.5h; Reflux; | 95.1% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
109-94-4
formic acid ethyl ester

-
-
50722-30-0
1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol for 40h; Bischler-Napieralski Reaction; Reflux; | 94% |
-
-
93-40-3
(3,4-Dimethoxyphenyl)acetic acid

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 17h; | 89% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
54417-53-7
(R)-tetrahydropapaverine hydrochloride

| Conditions | Yield |
|---|---|
| With N-acetyl-D-leucine; (R)-N-acetylphenylalanin In water; toluene; acetonitrile at 4 - 70℃; for 24.92h; Reagent/catalyst; | 83.25% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
938-18-1
mesitylene-2-carboxylic acid chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; | 83% |
-
-
50-00-0
formaldehyd

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
523-02-4, 4216-86-8, 6872-27-1, 13407-95-9
2,3,10,11-tetramethoxy-5,6,7,8,13,13a-hexahydroisoquinolino[3,2-a]isoquinoline

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 8h; | 80% |
| With acetic acid at 100℃; for 0.5h; Pictet-Spengler Synthesis; | 73% |
| In water Cyclization; Heating; | |
| In water for 30h; Heating; | 8.2 g |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
298-12-4
Glyoxilic acid

| Conditions | Yield |
|---|---|
| for 1h; Heating; | 80% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
61804-12-4
1,7-heptanediol dipropenoate

-
-
81165-43-7
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 7-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-heptyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 76% |
-
-
13048-33-4
hexamethylene glycol diacrylate

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
81182-08-3
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 6-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-hexyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 67% |
-
-
10526-04-2
1,6-hexanediol diacrylate

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
81165-44-8
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 8-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-octyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 60% |
-
-
4074-88-8
diethyleneglycol(diacrylate)

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
81165-45-9
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 2-(2-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-ethoxy)-ethyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 57% |
-
-
36840-85-4
pentamethylene 1,5-diacrylate

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
64228-78-0, 64493-10-3, 83285-74-9, 96687-52-4, 96687-53-5, 110817-20-4
pentamethylene bis(1-(3,4-dimethoxybenzyl)-3,4-dihydro-6,7-dimethoxy-1H-isoquinoline-2-propionate) dioxalate

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 51% |
-
-
24493-53-6
2-propenoic acid 1,3-propanediyl ester

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
81165-42-6
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 3-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-propyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 50% |
-
-
1070-70-8
1,4-Butanediol diacrylate

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
81182-07-2
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 4-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-butyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 48% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
64194-22-5
3-methyl-1,5-pentanediol dipropenoate

-
-
81165-48-2
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 5-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-3-methyl-pentyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 38% |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
36431-64-8
Acrylic acid 2-[4-(2-acryloyloxy-ethyl)-phenyl]-ethyl ester

-
-
81165-47-1
3-[1-(3,4-Dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionic acid 2-[4-(2-{3-[1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl]-propionyloxy}-ethyl)-phenyl]-ethyl ester; compound with oxalic acid

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 35% |
-
-
7647-01-0
hydrogenchloride

-
-
105-57-7
diethyl acetal

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
A
-
6899-65-6
(8R,13aR)-2,3,10,11-tetramethoxy-8-methyl-5,8,13,13a-tetrahydro-6H-isoquino[3,2-a]isoquinoline

-
-
7647-01-0
hydrogenchloride

-
-
109-87-5
Dimethoxymethane

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
7647-01-0
hydrogenchloride

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
A
-
74-87-3
methylene chloride

| Conditions | Yield |
|---|---|
| at 170 - 175℃; |

-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
52194-51-1, 52194-52-2, 52194-53-3, 52438-48-9, 57129-74-5, 62860-05-3, 62860-06-4, 65025-46-9, 120052-37-1
(+/-)-Xylopinin-trans-methiodid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8.2 g / H2O / 30 h / Heating 2: 122 mg / acetone; CHCl3 / 1 h / 20 °C View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8.2 g / H2O / 30 h / Heating 2: 1.85 g / HBr / 7 h / Heating View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8.2 g / H2O / 30 h / Heating 2: acetone; CHCl3 / 1 h / 20 °C View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8.2 g / H2O / 30 h / Heating 2: 71 mg / acetone; CHCl3 / 2.5 h / 20 °C View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8.2 g / H2O / 30 h / Heating 2: 290 mg / acetone; CHCl3 / 2.5 h / 20 °C View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
19716-66-6
pseudopalmatine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: H2O / Heating 2.1: 55 percent / mCPBA / CHCl3 / 20 °C 3.1: TFAA / CH2Cl2 / -30 - 20 °C 3.2: NaOAc / CH2Cl2; H2O / 0.5 h / 20 °C View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
6899-65-6, 7058-95-9, 57030-06-5, 58116-07-7, 58116-10-2, 58116-12-4, 79297-66-8
(+/-)-O-methylcorytenchirine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: H2O / Heating 2.1: 55 percent / mCPBA / CHCl3 / 20 °C 3.1: TFAA / CH2Cl2 / -30 - 20 °C 3.2: 49 percent / NaOAc / CH2Cl2; H2O / 0.5 h / 20 °C 4.1: 80 percent / methylmagnesium iodide / diethyl ether / 2 h / Heating View Scheme |
-
-
6429-04-5
1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline hydrochloride

-
-
332951-52-7
7,14-dehydroxylopinine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2O / Heating 2: 55 percent / mCPBA / CHCl3 / 20 °C 3: 50 percent Spectr. / acetyl chloride / 2 h / 25 °C View Scheme |
Tetrahydropapaverine hydrochloride Chemical Properties
Product Name: Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-,hydrochloride (1:1) (CAS NO.6429-04-5)
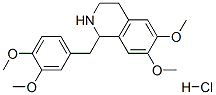
Molecular Formula: C20H25NO4.HCl
Molecular Weight: 379.8814g/mol
Mol File: 6429-04-5.mol
EINECS: 229-213-9
Boiling point: 475.8 °C at 760 mmHg
Flash Point: 202.7 °C
Enthalpy of Vaporization: 73.93 kJ/mol
Vapour Pressure: 3.23E-09 mmHg at 25°C
H-Bond Donor: 2
H-Bond Acceptor: 5
Structure Descriptors of Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-,hydrochloride (1:1) (CAS NO.6429-04-5):
IUPAC Name: 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride
Canonical SMILES: COC1=C(C=C(C=C1)CC2C3=CC(=C(C=C3CCN2)OC)OC)OC.Cl
InChI: InChI=1S/C20H25NO4.ClH/c1-22-17-6-5-13(10-18(17)23-2)9-16-15-12-20(25-4)19(24-3)11-14(15)7-8-21-16;/h5-6,10-12,16,21H,7-9H2,1-4H3;1H
InChIKey: VMPLLPIDRGXFTQ-UHFFFAOYSA-N
Classification Code: Drug / Therapeutic Agent
Tetrahydropapaverine hydrochloride Safety Profile
Safety Information of Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-,hydrochloride (1:1) (CAS NO.6429-04-5):
Hazard Codes: T
Tetrahydropapaverine hydrochloride Specification
Isoquinoline,1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-,hydrochloride (1:1) , its CAS NO. is 6429-04-5, the synonyms are 1,2,3,4-Tetrahydro-6,7-dimethoxy-1-veratryl-isoquinoline hydrochloride ; Papaverine, 1,2,3,4-tetrahydro-, hydrochloride ; Tetrahydropapaverine hydrochloride ; 1-(3,4-Dimethoxybenzyl)-1,2,3,4-tetrahydro-6,7-
dimethoxyisoquinolinium chloride ; Isoquinoline, 1,2,3,4-tetrahydro-6,7-dimethoxy-1-veratryl-, hydrochloride .
Related Products
- Tetrahydropapaverine hydrochloride
- 6429-10-3
- 642-91-1
- 6429-12-5
- 64291-41-4
- 642928-07-2
- 64294-88-8
- 64294-95-7
- 64296-23-7
- 64297-16-1
- 64297-64-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View