-
Name
Tetrahydrophthalimide
- EINECS 201-602-8
- CAS No. 85-40-5
- Article Data15
- CAS DataBase
- Density 1.228
- Solubility 12.2g/L at 20℃
- Melting Point 132-140 ºC
- Formula C8H9 N O2
- Boiling Point 337 ºC
- Molecular Weight 151.165
- Flash Point 165 ºC
- Transport Information
- Appearance white crystalline powder
- Safety Experimental reproductive effects. When heated to decomposition it emits toxic vapors of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-Cyclohexene-1,2-dicarboximide(6CI,7CI,8CI); 1,2,3,6-Tetrahydrophthalimide;3a,4,7,7a-Tetrahydro-1H-isoindole-1,3(2H)-dione;3a,4,7,7a-Tetrahydroisoindole-1,3-dione; NSC 59011; Tetrahydrophthalic acidimide; Tetrahydrophthalimide; D4-Tetrahydrophthalimide
- PSA 46.17000
- LogP 0.55400
Synthetic route

| Conditions | Yield |
|---|---|
| In 1,3,5-trimethyl-benzene at 170℃; for 2.5h; Temperature; Solvent; Large scale; | 95% |

| Conditions | Yield |
|---|---|
| With choline chloride; urea at 140℃; for 1h; Green chemistry; | 81% |
| With complex<3THF*Mg2Cl2O*TiNCO>6 In pyridine Heating; Yield given; |

-
-
85-40-5
1,2,3,6-tertahydrophthalimide

| Conditions | Yield |
|---|---|
| With ammonia at 180℃; | 74% |
-
-
42027-93-0
N-Hydroxymethyl-cis-4-cyclohexene-1,2-dicarboximide

-
-
85-40-5
1,2,3,6-tertahydrophthalimide

| Conditions | Yield |
|---|---|
| at 100℃; Substitution; Thermolysis; |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
83809-94-3
1,10-bis(methoxymethyl)-1,10-diaza-18-crown-6

-
-
91043-68-4
C30H44N4O8

| Conditions | Yield |
|---|---|
| In benzene reflux; 20 deg C, 2 h; | 99% |

| Conditions | Yield |
|---|---|
| In ethanol for 2.5h; Addition; Heating; | 99% |
-
-
109-65-9
1-bromo-butane

-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
2021-19-4
2-butyl-3a,4,7,7a-tetrahydro-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In acetone for 24h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| With [Ru4H6(p-cumene)4]Cl2; hydrogen In water at 90℃; under 45003.6 Torr; for 22h; | 97% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
54512-75-3
1-bromo-5-chloropentane

-
-
929224-25-9
2-(5-chloropentyl)-3a,4,7,7a-tetrahydro-isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 60h; | 92% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
928-96-1
(Z)-3-Hexen-1-ol

-
-
82478-58-8
((Z)-2-Hex-3-enyl)-3a,4,7,7a-tetrahydro-isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| With dimethyl azodicarboxylate; triphenylphosphine In tetrahydrofuran Ambient temperature; overnight; | 90% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
141-32-2
acrylic acid n-butyl ester

-
-
1252935-87-7
butyl 3-(3a,4,7,7a-tetrahydro-1,3-dioxo-1H-isoindol-2(3H)-yl)propanoate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammomium bromide at 100℃; for 1.5h; aza-Michael addition; Neat (no solvent); | 90% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; | 87% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
623-91-6
diethyl Fumarate

-
-
1252935-79-7
diethyl 2-(3a,4,7,7a-tetrahydro-1,3-dioxo-1H-isoindol-2(3H)-yl)succinate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammomium bromide at 100℃; for 2.5h; aza-Michael addition; Neat (no solvent); | 85% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 50℃; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| With oxygen; copper(l) chloride In acetonitrile at 50℃; for 24h; Green chemistry; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 82% |
-
-
763-32-6
2-methyl-1-buten-4-ol

-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
82478-57-7
2-(3-Methyl-but-3-enyl)-3a,4,7,7a-tetrahydro-isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| With dimethyl azodicarboxylate; triphenylphosphine In tetrahydrofuran Ambient temperature; overnight; | 81% |
-
-
182208-57-7
3-(1,2,4,4a,5,6-hexahydro-pyrazino[1,2-a]quinolin-3-yl)-propylamine

-
-
85-40-5
1,2,3,6-tertahydrophthalimide

| Conditions | Yield |
|---|---|
| In toluene Heating / reflux; | 80% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
927-74-2
1-butyn-4-ol

-
-
82478-59-9
2-But-3-ynyl-3a,4,7,7a-tetrahydro-isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| With dimethyl azodicarboxylate; triphenylphosphine In tetrahydrofuran Ambient temperature; overnight; | 78% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
62360-00-3
(Z)-2-fluoro-3-phenylprop-2-enyl acetate

-
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; bis(η3-allyl-μ-chloropalladium(II)); caesium carbonate In toluene at 80℃; for 16h; Inert atmosphere; regioselective reaction; | 78% |

-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
24424-99-5
di-tert-butyl dicarbonate

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3,6-tertahydrophthalimide With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 60℃; for 18h; Stage #2: di-tert-butyl dicarbonate With triethylamine In dichloromethane at 0 - 20℃; for 21.5h; | 77.3% |

| Conditions | Yield |
|---|---|
| With oxygen; copper(l) chloride In acetonitrile at 50℃; for 24h; Green chemistry; regioselective reaction; | 77% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
14595-35-8
di-n-propyl fumarate

-
-
1252935-80-0
dipropyl 2-(3a,4,7,7a-tetrahydro-1,3-dioxo-1H-isoindol-2(3H)-yl)succinate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammomium bromide at 100℃; for 3.5h; aza-Michael addition; Neat (no solvent); | 76% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
474925-37-6
(3aH,7aS)-tert-butyl 3a,4,7,7a-tetrahydro-1H-isoindole-2(3H)-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3,6-tertahydrophthalimide With lithium aluminium tetrahydride In tetrahydrofuran for 24h; Heating; Stage #2: di-tert-butyl dicarbonate In dichloromethane for 1h; Further stages.; | 73% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
109-64-8
1,3-dibromo-propane

-
-
56401-24-2
2-(3-bromopropyl)-3a,4.7,7a-tetrahydro-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In acetone at 40℃; for 8h; | 72% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
105-75-9
dibutyl fumarate

-
-
1252935-81-1
dibutyl 2-(3a,4,7,7a-tetrahydro-1,3-dioxo-1H-isoindol-2(3H)-yl)succinate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammomium bromide at 100℃; for 3.5h; aza-Michael addition; Neat (no solvent); | 72% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
20314-74-3
fumaric acid dipentyl ester

-
-
1252935-82-2
dipentyl 2-(3a,4,7,7a-tetrahydro-1,3-dioxo-1H-isoindol-2(3H)-yl)succinate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammomium bromide at 100℃; for 4h; aza-Michael addition; Neat (no solvent); | 70% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine | 69% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
19139-31-2
dihexyl fumarate

-
-
1252935-83-3
dihexyl 2-(3a,4,7,7a-tetrahydro-1,3-dioxo-1H-isoindol-2(3H)-yl)succinate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammomium bromide at 100℃; for 4.5h; aza-Michael addition; Neat (no solvent); | 68% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
598-31-2
1-bromoacetone

-
-
54399-02-9
1-(Δ4-tetrahydrophthalimido)propan-2-one

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 2h; Heating; | 64% |
-
-
85-40-5
1,2,3,6-tertahydrophthalimide

-
-
20314-73-2
diheptyl fumarate

-
-
1252935-84-4
diheptyl 2-(3a,4,7,7a-tetrahydro-1,3-dioxo-1H-isoindol-2(3H)-yl)succinate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrabutylammomium bromide at 100℃; for 5h; aza-Michael addition; Neat (no solvent); | 63% |
Tetrahydrophthalimide Chemical Properties
IUPAC Name: 3A,4,7,7A-Tetrahydroisoindole-1,3-dione (85-40-5)
Synonyms: 3A,4,7,7A-Tetrahydro-1h-isoindole-3(2h)-dione ; 3A,4,7,7A-tetrahydro-isoindole-3-dione ; 3-Alpha,4,7,7-alpha-tetrahydro-1h-isoindole-3(2h)-dione ; Delta(4)-tetrahydrophthalimide ; Delta(sup4)-tetrahydrophthalimide ; Tetrahydrophthalimide ; 4-Cyclohexene-1,2-dicarboximide ; 1,2,3,6-Tetrahydrophthalimide
CAS: 85-40-5
MF: C8H9NO2
MW: 151.16
MS: 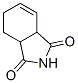
EINECS: 201-602-8
Product Categories:
Mol File: 85-40-5.mol
Surface Tension: 42.1 dyne/cm
Density: 1.228 g/cm3
Flash Point: 165 °C
Melting point: 132-140°C
Enthalpy of Vaporization: 57.99 kJ/mol
Boiling Point: 336.8 °C at 760 mmHg
Vapour Pressure: 0.00011 mmHg at 25°C
Tetrahydrophthalimide Consensus Reports
Reported in EPA TSCA Inventory.
Related Products
- Tetrahydrophthalimide
- 854056-07-8
- 854067-23-5
- 85407-92-7
- 85408-10-2
- 85408-32-8
- 85409-09-2
- 85409-25-2
- 85409-36-5
- 85409-38-7
- 85409-42-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View