-
Name
TRIS(2-CYANOETHYL)PHOSPHINE
- EINECS 223-687-0
- CAS No. 4023-53-4
- Article Data12
- CAS DataBase
- Density
- Solubility Soluble in DMSO and H2O
- Melting Point 97-98°C
- Formula C9H12N3P
- Boiling Point 426.9 °C at 760 mmHg
- Molecular Weight 193.188
- Flash Point 212 °C
- Transport Information UN 3464
- Appearance white crystalline
- Safety 36/37/39-45-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Propionitrile,3,3',3'-phosphinidynetri- (7CI);Propionitrile, 3,3',3''-phosphinidynetri-(6CI,8CI);NSC 41940;Phosphine, tris(2-cyanoethyl)-;Tris(2-cyanoethyl)phosphine;Tris(b-cyanoethyl)phosphine;
- PSA 84.96000
- LogP 2.20934
Synthetic route
-
-
107-13-1
acrylonitrile

-
A
-
4023-49-8
bis(2-cyanoethyl)phosphine

-
B
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
C
-
1439-41-4
tris(2-cyanoethyl)phosphine oxide

| Conditions | Yield |
|---|---|
| With potassium hydroxide; phosphan In water; acetonitrile for 1h; Ambient temperature; | A 15 % Spectr. B 45% C 15 % Spectr. |
| With phosphorus; potassium hydroxide In water; acetonitrile at 20 - 70℃; for 0.166667h; Irradiation; sonication; | A 10 % Spectr. B 30 % Spectr. C 60 % Spectr. |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; phosphan; acetonitrile | |
| With phosphan |
-
-
107-13-1
acrylonitrile

-
A
-
4023-49-8
bis(2-cyanoethyl)phosphine

-
B
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
C
-
6783-71-7
3-phosphanyl-propionitrile

| Conditions | Yield |
|---|---|
| With {(tris(cyanomethyl)phosphine)3platinum}; phosphan In acetonitrile at 20℃; for 1h; Product distribution; Mechanism; | |
| With aluminum oxide; potassium hydroxide; phosphan Product distribution; influence of varying degrees of dryness of solid support; | |
| With phosphan; In acetonitrile |


| Conditions | Yield |
|---|---|
| In acetonitrile |
-
-
107-13-1
acrylonitrile

-
-
75-05-8
acetonitrile

-
A
-
4023-49-8
bis(2-cyanoethyl)phosphine

-
B
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
C
-
6783-71-7
3-phosphanyl-propionitrile

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
188638-31-5
[Pt(C8H12)(C3H4NO)2]

-
-
188638-33-7
[Pt(C3H4NO)2(P(CH2CH2CN)3)2]

| Conditions | Yield |
|---|---|
| In dichloromethane pptn. with light petroleum, filtration, washing (light petroleum), drying (vac.); elem. anal.; | 100% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
200112-75-0
3-(perfluorooctyl)propyl iodide

| Conditions | Yield |
|---|---|
| at 130 - 160℃; for 11h; | 99.8% |

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
337380-82-2
Fe2(CO)5(P(CH2CH2CN)3)(HCCHC6H5)P(C6H5)2

| Conditions | Yield |
|---|---|
| In toluene byproducts: CO; (N2); refluxing a soln. of iron complex and phosphine in toluene for 20 min, cooling to room temp.; chromy (deactivated Al2O3, Et2O/light petroleum 2:3); elem. anal.; | 98% |
-
-
15617-18-2, 39958-10-6, 14220-64-5
bis(benzonitrile)palladium(II) dichloride

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
204461-54-1, 68494-75-7
trans-PdCl2(tris(cyanoethyl)phosphine)2

| Conditions | Yield |
|---|---|
| In dichloromethane N2-atmosphere; dropwise addn. of 2 equiv. of phosphine to Pd-complex soln., stirring for 10 min (pptn.); filtration, washing (CH2Cl2), drying (vac.); elem. anal.; | 98% |

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
204391-74-2
cis-PtMe2(tris(cyanoethyl)phosphine)2

| Conditions | Yield |
|---|---|
| In acetone N2-atmosphere; dropwise addn. of ligand soln. to 0.5 equiv. of Pt-complex soln., stirring for 10 min; concn., pptn. on Et2O addn., filtration, washing (Et2O), drying (vac.); elem. anal.; | 97% |
-
-
39929-21-0
(tetrahydrothiophene)gold(I) chloride

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
148100-44-1
{tris(2-cyanoethyl)phosphine}gold(I) chloride

| Conditions | Yield |
|---|---|
| In dichloromethane; acetonitrile N2; equimolar amounts; stirring for 1 h at room temperature;; precipitation upon addn. of diethyl ether;; | 95% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
156457-81-7
bis(4-formylphenyl) azidothiophosphonate

| Conditions | Yield |
|---|---|
| In acetonitrile Ambient temperature; | 94% |
-
-
12080-32-9
dichloro(1,5-cyclooctadiene)platinum(ll)

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
20699-88-1
trans-PtCl2(tris(cyanoethyl)phosphine)2

| Conditions | Yield |
|---|---|
| In dichloromethane N2-atmosphere; dropwise addn. of ligand soln. to 0.5 equiv. of Pt-complex soln. (pptn.); filtration, washing (CH2Cl2), drying (vac.); | 93% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In acetone N2-atmosphere; stirring stoich. amts. for 10 min; concn., pptn. on Et2O addn., filtration, washing (Et2O), drying (vac.); elem. anal.; | 93% |
-
-
12092-47-6
di-μ-chloro-bis(1,5-cyclooctadiene)dirhodium

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
204391-82-2
RhCl(tris(cyanoethyl)phosphine)(COD)

| Conditions | Yield |
|---|---|
| In acetone N2-atmosphere; stirring Rh-complex with 2 equiv. of phosphine for 15 min; concn., pptn. on Et2O addn.; elem. anal.; | 93% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
204461-54-1, 68494-75-7
trans-PdCl2(tris(cyanoethyl)phosphine)2

-
-
200437-22-5
Pd(tris(cyanoethyl)phosphine)3

| Conditions | Yield |
|---|---|
| With LiOMe In methanol; ethanol N2-atmosphere; addn. of 4 equiv. of 0.1 M LiOMe (in MeOH) to EtOH, then addn. of 1 equiv. of Pd-complex and 2 equiv. phosphine, refluxing for 2 h (pptn.); filtration, washing (Et2O), drying (vac.); elem. anal.; | 92% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
7474-83-1
3-bromo-1,2-naphthoquinone

-
A
-
1439-41-4
tris(2-cyanoethyl)phosphine oxide

-
B
-
61978-07-2
3-bromo-1,2-dihydroxynaphthalene

| Conditions | Yield |
|---|---|
| With water In dichloromethane for 24h; Inert atmosphere; | A 92% B 80% |

-
-
75-77-4
chloro-trimethyl-silane

-
-
14221-06-8, 744215-74-5
tetrakis(acetato)dimolybdenum(II)

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In toluene N2-atmosphere; dropwise addn. of excess Me3SiCl to stoich. mixt. of Mo-compd. and ligand, stirring at room temp. for >= 2 d (pptn.); collection (filtration), washing (PhMe, 40°C; hexanes at room temp.; Et2O), drying (vac., room temp., >=12 h); elem. anal.; | 90% |
| In tetrahydrofuran N2-atmosphere; dropwise addn. of excess Me3SiCl to stoich. mixt. of Mo-compd. and ligand, stirring at room temp. for >= 2 d (pptn.); collection (filtration), washing (PhMe, 40°C; hexanes at room temp.; Et2O), drying (vac., room temp., >=12 h); | 90% |
| In benzene N2-atmosphere; dropwise addn. of excess Me3SiCl to stoich. mixt. of Mo-compd. and ligand, stirring at room temp. for >= 2 d (pptn.); collection (filtration), washing (PhMe, 40°C; hexanes at room temp.; Et2O), drying (vac., room temp., >=12 h); | 90% |


-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In ethanol; acetone EtOH:acetone:triethyl orthoformate=6:5:1, equimolar amts. of metal salts, excess of ligand, ambient temp. (pptn., then dissoln. of Ni-complex, crystn. of mixt. of products on slow evapn.); mixt. of products not separated; | A 8% B 90% C 2% |

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
101190-22-1
{Co(CNC(CH3)3)3(P(C2H4CN)3)2}(1+)*(ClO4)(1-)={Co(CNC(CH3)3)3(P(C2H4CN)3)2}(ClO4)

| Conditions | Yield |
|---|---|
| In acetonitrile at 0°C;; recrystallization from methylene dichloride and ether, elem. anal.; | 89% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
51805-45-9
tris-(2-carboxyethyl)-phosphine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 2h; Heating; | 88% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
132199-05-4
{(tris(cyanomethyl)phosphine)3platinum}

| Conditions | Yield |
|---|---|
| In acetone; toluene N2-atmosphere; dropwise addn. of 3 equiv. of phosphine (in Me2CO) to Pt-complex soln. (in PhMe, pptn.), stirring for 3 h; filtration, dissoln. in Me2CO, pptn. on PhMe addn.; elem. anal.; | 86% |
-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
172915-72-9
bis[tris(2-cyanoethyl)phosphine]silver(I) nitrate

| Conditions | Yield |
|---|---|
| In ethanol; acetonitrile addn. of AgNO3 (in hot MeCN) to 2 equiv. of ligand (in warm EtOH); crystn. on slow cooling (in dark); elem. anal.; | 85% |

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
80510-03-8
Tris(pyrazolyl)methane

| Conditions | Yield |
|---|---|
| Stage #1: tetrakis(acetonitrile)copper(I)tetrafluoroborate; tris(2-cyanoethyl)phosphine In acetonitrile for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: Tris(pyrazolyl)methane In acetonitrile for 4h; Schlenk technique; Inert atmosphere; | 85% |


-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In acetone addn. of phosphine soln. to stirred Ni complex soln. under N2; stirring, 4h; concentratin; addn. of hexane; pptn.; filtration; washing (Et2O); elem. anal.; | 84% |

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In acetone refluxing (1 h); pptn. on concn., addn. of MeOH and cooling; elem. anal.; | 83% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 0.333333h; Reflux; Inert atmosphere; | 83% |
-
-
64443-05-6
tetrakis(actonitrile)copper(I) hexafluorophosphate

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In acetonitrile for 1h; Reflux; Inert atmosphere; | 83% |

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In acetone N2-atmosphere; stirring Rh-complex with 4 equiv. of phosphine for 40 min; evapn.; elem. anal.; | 82% |

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
204391-83-3
IrCl(tris(cyanoethyl)phosphine)(COD)

| Conditions | Yield |
|---|---|
| In acetone N2-atmosphere; stirring Ir-complex with 2 equiv. of phosphine for 15 min; concn., pptn. on Et2O addn.; elem. anal.; | 82% |
-
-
12080-32-9
dichloro(1,5-cyclooctadiene)platinum(ll)

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

-
-
111046-55-0
PtCl2(CO)P(CH2CH2CN)3

| Conditions | Yield |
|---|---|
| With carbon monoxide In chloroform CO was bubbled through a soln. of Pt-complex in CHCl3 for 1 h, a soln. of the phosphine in hot CHCl3 was added dropwise over 30 min with stirring; ppt. was washed with CHCl3, recrystd. from acetone-propan-2-ol; elem. anal.; | 81% |
-
-
55272-36-1
[RuCl(η(5)-C5H4Me)(PPh3)2]

-
-
4023-53-4
tris(2-cyanoethyl)phosphine

| Conditions | Yield |
|---|---|
| In acetone N2 atmosphere; refluxing (1 h); concn. of aoln., pptn. (addn. of chloroform); elem. anal.; | 79% |
| In toluene N2 atmosphere; refluxing (1.5 h), pptn.; dissolution (acetone), repptn. (addn. of chloroform); elem. anal.; | 79% |
Tris(2-cyanoethyl)phosphine Specification
The CAS registry number of Propanenitrile,3,3',3''-phosphinidynetris- is 4023-53-4. It belongs to the product category of Tertiary Phosphines. This chemical is also named as 3,3',3''-Phosphinetriyltripropanenitrile. Its EINECS registry number is 223-687-0. In addition, its molecular formula is C9H12N3P and molecular weight is 193.18. Its IUPAC name is called 3-[bis(2-cyanoethyl)phosphanyl]propanenitrile.
Physical properties about Propanenitrile,3,3',3''-phosphinidynetris- are: (1)ACD/LogP: -0.25; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.25; (4)ACD/LogD (pH 7.4): -0.25; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 17.43; (8)ACD/KOC (pH 7.4): 17.43; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 6; (12)Flash Point: 212 °C; (13)Enthalpy of Vaporization: 68.17 kJ/mol; (14)Boiling Point: 426.9 °C at 760 mmHg.
Preparation: this chemical can be prepared by acrylonitrile. This reaction will need reagents PH3, KOH and solvents acetonitrile, H2O. The reaction time is 1 hour at ambient temperature. The yield is about 45 %.
.png)
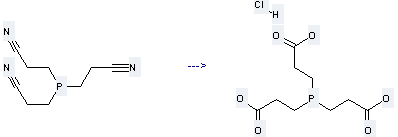
Uses of Propanenitrile,3,3',3''-phosphinidynetris-: it can be used to produce 3,3',3''-phosphanetriyl-tri-propionic acid and hydrochloride by heating. It will need reagent conc. aq. HCl with reaction time of 2 hours. The yield is about 88 %.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: N#CCCP(CCC#N)CCC#N
(2)InChI: InChI=1/C9H12N3P/c10-4-1-7-13(8-2-5-11)9-3-6-12/h1-3,7-9H2
(3)InChIKey: CHZAMJVESILJGH-UHFFFAOYAA
Related Products
- Tris(2-cyanoethyl)phosphine
- 4023-65-8
- 4023-79-4
- 4023-80-7
- 402-41-5
- 402-42-6
- 402-43-7
- 402-44-8
- 40244-90-4
- 40245-26-9
- 402-45-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View