-
Name
Urocanic acid
- EINECS 203-258-4
- CAS No. 104-98-3
- Article Data8
- CAS DataBase
- Density 1.43 g/cm3
- Solubility SLIGHTLY SOLUBLE
- Melting Point 226-228 °C(lit.)
- Formula C6H6N2O2
- Boiling Point 456.9 °C at 760 mmHg
- Molecular Weight 138.126
- Flash Point 230.1 °C
- Transport Information
- Appearance white to beige fine powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-Imidazolylacrylic acid;5-Imidazoleacrylicacid;NSC 66357;Urocaninic acid;3-(1H-Imidazol-4-yl)acrylic acid;3-(4-Imidazolyl)acrylic acid;3-(1H-Imidazol-4-yl)-2-propenoic acid;Imidazole-4-acrylic acid (8CI);2-Propenoicacid, 3-(1H-imidazol-4-yl)- (9CI);
- PSA 65.98000
- LogP 0.50750
Synthetic route

| Conditions | Yield |
|---|---|
| With histidase EC 4.3.1.3; Tris buffer In water at 37℃; for 24h; pH=9.0; | |
| With histidase Enzymatic reaction; |
-
-
104-98-3
urocanic acid

-
-
49549-75-9
4-(3-hydroxypropyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride | 100% |
-
-
67-56-1
methanol

-
-
104-98-3
urocanic acid

-
-
160073-02-9, 52363-40-3
methyl 3-(imidazol-4(5)-yl)-2-propenoate

| Conditions | Yield |
|---|---|
| With thionyl chloride | 98% |
| With hydrogenchloride Reflux; | 94% |
| With sulfuric acid; magnesium sulfate Fischer esterification; Reflux; | 93% |
-
-
67-56-1
methanol

-
-
104-98-3
urocanic acid

-
-
53958-94-4
3-(1H-imidazol-4-yl)propionic acid methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: urocanic acid With hydrogenchloride; palladium 10% on activated carbon; hydrogen In methanol; water at 45℃; under 760.051 Torr; for 96h; Large scale; Stage #2: methanol With hydrogenchloride at 45℃; Large scale; | 98% |

| Conditions | Yield |
|---|---|
| at 250℃; | 93% |
| at 110℃; | 71% |
| at 240℃; under 0.0165017 Torr; for 6h; | 66% |
-
-
67-56-1
methanol

-
-
104-98-3
urocanic acid

-
-
52838-22-9
methyl 3-[1H-imidazol-4-yl]propenoate hydrochloride salt

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0℃; for 2h; | 87% |
-
-
57260-73-8
N-BOC-1,2-diaminoethane

-
-
104-98-3
urocanic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: urocanic acid With palladium on activated charcoal; hydrogen In methanol at 25℃; Stage #2: ethanol With sulfuric acid In methanol Reflux; Inert atmosphere; | 82% |
| Stage #1: urocanic acid With palladium on activated charcoal; hydrogen In methanol at 25℃; Stage #2: ethanol With sulfuric acid Reflux; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol; urocanic acid With thionyl chloride for 16h; Reflux; Stage #2: With sodium carbonate In water; ethyl acetate | 81% |
-
-
70-34-8
2,4-Dinitrofluorobenzene

-
-
104-98-3
urocanic acid

-
-
507444-19-1
3-[1-(2,4-dinitrophenyl)-1H-imidazol-4-yl]propenoic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In methanol; water at 20℃; for 16h; Substitution; | 76% |

| Conditions | Yield |
|---|---|
| In ethanol; toluene for 24h; Reflux; Dean-Stark; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: urocanic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 0.0833333h; Stage #2: 2,2,2-trifluoroethanol In dichloromethane at 20℃; for 18h; | 64% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 80℃; for 18h; Sealed tube; Inert atmosphere; | 64% |

| Conditions | Yield |
|---|---|
| In ethanol; toluene for 24h; Reflux; Dean-Stark; | 60% |

| Conditions | Yield |
|---|---|
| In ethanol; water High Pressure; equimol., heated at 433 K for 72 h; slowly cooled to room temp. (72 h), crysts. collected; elem. anal.; | 48% |

-
-
174221-86-4
5'-O-dimethoxytrityl-2'-amino-2'-deoxyuridine

-
-
104-98-3
urocanic acid

| Conditions | Yield |
|---|---|
| Stage #1: urocanic acid With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; Stage #2: 5'-O-dimethoxytrityl-2'-amino-2'-deoxyuridine In N,N-dimethyl-formamide at 20℃; Further stages.; | 45% |

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In N,N-dimethyl-formamide at -5 - 20℃; for 12h; | 45% |

| Conditions | Yield |
|---|---|
| With iodine In neat (no solvent) at 20 - 23℃; for 8h; | 25% |

| Conditions | Yield |
|---|---|
| In methanol; water High Pressure; stoich., ligand in CH3OH mixed dropwise with soln. of Cu salt in h2O, heated in an autoclave at 90°C for 3 d; isolated, elem. anal.; | 11% |
-
-
947699-91-4
N1-(2-aminoethyl)-N2-(7-chloroquinolin-4-yl)-N1-methylethane-1,2-diamine

-
-
104-98-3
urocanic acid

| Conditions | Yield |
|---|---|
| Stage #1: urocanic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide for 2h; Inert atmosphere; Stage #2: N1-(2-aminoethyl)-N2-(7-chloroquinolin-4-yl)-N1-methylethane-1,2-diamine In N,N-dimethyl-formamide for 21h; Inert atmosphere; | 8% |

| Conditions | Yield |
|---|---|
| With pyridine In water for 0.0833333h; |

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
104-98-3
urocanic acid

-
-
1006066-10-9
3-(1-tert-butoxycarbonyl-imidazol-4-yl)-acrylic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; |
-
-
104-98-3
urocanic acid

-
-
112606-55-0
C26H22N2O2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / SOCl2 2: Et3N / dimethylformamide View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; magnesium sulfate / Reflux 2: triethylamine / N,N-dimethyl-formamide View Scheme |
-
-
104-98-3
urocanic acid

-
-
226930-86-5
4-chloroallyl-1-tritylimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 98 percent / SOCl2 2: Et3N / dimethylformamide 3: 75 percent / LiAlH4 / tetrahydrofuran 4: 75 percent / SOCl2 / tetrahydrofuran / 1 h View Scheme |
-
-
104-98-3
urocanic acid

-
-
500782-79-6
C44H38N4O3

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 98 percent / SOCl2 2: Et3N / dimethylformamide 3: 75 percent / LiAlH4 / tetrahydrofuran 4: 75 percent / SOCl2 / tetrahydrofuran / 1 h 5: 21 percent / NaH / tetrahydrofuran / 2 h / 60 °C View Scheme |
-
-
104-98-3
urocanic acid

-
-
500782-80-9
C36H36N4O

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / SOCl2 2: Et3N / dimethylformamide 3: 75 percent / LiAlH4 / tetrahydrofuran 4: 75 percent / SOCl2 / tetrahydrofuran / 1 h 5: 21 percent / NaH / tetrahydrofuran / 2 h / 60 °C 6: 99 percent / H2 / Pd/C / methanol; ethyl acetate / atmospheric pressure View Scheme |
-
-
104-98-3
urocanic acid

-
-
500782-81-0
C44H49N5O4

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 98 percent / SOCl2 2: Et3N / dimethylformamide 3: 75 percent / LiAlH4 / tetrahydrofuran 4: 75 percent / SOCl2 / tetrahydrofuran / 1 h 5: 21 percent / NaH / tetrahydrofuran / 2 h / 60 °C 6: 99 percent / H2 / Pd/C / methanol; ethyl acetate / atmospheric pressure 7: 85 percent / CH2Cl2 / 5 h / 0 - 20 °C View Scheme |
-
-
104-98-3
urocanic acid

-
-
501010-03-3
C25H35N5O4

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 98 percent / SOCl2 2: Et3N / dimethylformamide 3: 75 percent / LiAlH4 / tetrahydrofuran 4: 75 percent / SOCl2 / tetrahydrofuran / 1 h 5: 21 percent / NaH / tetrahydrofuran / 2 h / 60 °C 6: 99 percent / H2 / Pd/C / methanol; ethyl acetate / atmospheric pressure 7: 85 percent / CH2Cl2 / 5 h / 0 - 20 °C 8: 90 percent / TFA; triethylsilane / CH2Cl2 / 1 h / 20 °C View Scheme |
-
-
104-98-3
urocanic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 98 percent / SOCl2 2: Et3N / dimethylformamide 3: 75 percent / LiAlH4 / tetrahydrofuran 4: 75 percent / SOCl2 / tetrahydrofuran / 1 h 5: 21 percent / NaH / tetrahydrofuran / 2 h / 60 °C 6: 99 percent / H2 / Pd/C / methanol; ethyl acetate / atmospheric pressure 7: 85 percent / CH2Cl2 / 5 h / 0 - 20 °C 8: 90 percent / TFA; triethylsilane / CH2Cl2 / 1 h / 20 °C 9: 85 percent / aq. NaOH / methanol / 1 h / 20 °C View Scheme |
UROCANIC ACID Chemical Properties
Chemical Name: Urocanic acid
Molecular Formula: C6H6N2O2
molecular structure:
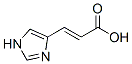
Formula Weight: 138.12
CAS No.: 104-98-3
CBNumber: CB8750635
Synonyms:3-(1h-imidazol-4-yl)-2-propenoicaci;3-(1h-imidazol-4-yl)-2-propenoicacid;3-imidazol-4-ylacrylic;3-imidazol-4-ylacrylicacid;5-imidazoleacrylicacid;imidazoleacrylicacid;3-(1H-IMIDAZOL-4-YL)ACRYLIC ACID;3-(4-IMIDAZOLY)ACRYLIC ACID
storage temp:2-8°C
mp :226-228 °C
slightly soluble in water
Urocanic acid is an intermediate in the catabolism of L-histidine.
Urocanic acid was detected in animal sweat and skin where, among other possible functions, it acts as an endogenous sunscreen or photoprotectant against UVB-induced DNA damage. When exposed to UVB irradiation, trans-urocanic acid is converted in vitro and in vivo to the cis isomer. The cis form is known to activate suppressor T cellsUrocanic acid is found predominantly in the stratum corneum of the skin and it is likely that most of it is derived from filaggrin catabolism (a histidine-rich protein).
UROCANIC ACID History
UROCANIC ACID Toxicity Data With Reference
| 1. | dni-hmn:oth 1 mmol/L | JIDEAE Journal of Investigative Dermatology. 65 (1975),400. | ||
| 2. | ivn-mus LD50:100 mg/kg | 85GDA2 CRC Handbook of Antibiotic Compounds. 5 (1981),81. |
UROCANIC ACID Consensus Reports
UROCANIC ACID Safety Profile
Related Products
- UROCANIC ACID
- 10498-35-8
- 104983-85-9
- 104986-28-9
- 104987-11-3
- 104987-12-4
- 104989-09-5
- 104996-63-6
- 10500-34-2
- 10500-57-9
- 105005-94-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View