-
Name
Undecan-4-olide
- EINECS 203-225-4
- CAS No. 104-67-6
- Article Data36
- CAS DataBase
- Density 0.9425 g/cm3
- Solubility 158mg/L at 20℃
- Melting Point 164~166℃
- Formula C11H20O2
- Boiling Point 286 °C at 760 mmHg
- Molecular Weight 184.279
- Flash Point 112.7 °C
- Transport Information
- Appearance Colourless oily liquid
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Undecanoicacid, 4-hydroxy-, g-lactone(6CI,7CI);4-Hydroxyundecanoic acid lactone;4-Undecanolide;5-Heptyldihydro-2(3H)-furanone;5-Heptyltetrahydro-2-furanone;NSC 406421;NSC46118;NSC 76413;Neutralizing agent 350120-1;Peach lactone;Peche Pure;Persicol;g-(n-Heptyl)-g-butyrolactone;g-Heptyl-g-butyrolactone;g-Heptylbutyrolactone;g-Undecalactone;g-Undecanolactone;g-Undecanolide;g-n-Heptylbutyrolactone;
- PSA 26.30000
- LogP 3.05250
Synthetic route

| Conditions | Yield |
|---|---|
| With 2Zn(2+)*(CH3)3NC2H4OH(1+)*5Cl(1-)=[HOC2H4N(CH3)3]Zn2Cl5 at 130℃; for 8h; | 94% |
| With silver trifluoromethanesulfonate In chlorobenzene at 130℃; for 20h; Inert atmosphere; regioselective reaction; | 71% |
-
-
22127-86-2
undec-1-yn-4-ol

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; C21H12F9P*C8H18NO4S2(1-)*Au(1+); 3-chloro-benzenecarboperoxoic acid In 1,2-dichloro-ethane at 20℃; for 5h; Reagent/catalyst; | 90% |
| Multi-step reaction with 2 steps 1.1: n-butyllithium / diethyl ether; hexane / 0.75 h / 0 - 20 °C 1.2: 0 - 20 °C 2.1: C21H12F9P*C8H18NO4S2(1-)*Au(1+); methanesulfonic acid / chloroform-d1 / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With aluminum oxide; sodium bromite In acetonitrile for 3h; Ambient temperature; | 71% |
| With 4 A molecular sieve; polymer intercalated (RuCl2(PPh3)3); 4-methylmorpholine N-oxide In acetone at 30℃; for 2h; | 79 % Chromat. |
| Cp*RuCl(Ph2P(CH2)2NH2-κ2-P,N); potassium tert-butylate In acetone at 30℃; for 1h; | 95 % Spectr. |
-
-
114702-12-4
4-Hydroxy-undec-2-ynoic acid ethyl ester

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With formic acid; tributyl-amine; Pd(OAc)2*3(PPh2) In N,N-dimethyl-formamide at 60℃; for 7h; | 64% |
-
-
50915-95-2
1-(cyclopropylidene)octane

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With trifluoroacetyl peroxide In dichloromethane at 0℃; for 6h; | 87% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide at 95 - 180℃; Large scale; | 1715 kg |
| With di-tert-butyl peroxide at 20 - 180℃; | 168 g |
-
-
33566-59-5
methyl 4-oxo-dodecanoate

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; disodium hydrogenphosphate In methanol 1.) 0 deg C, 5 min, 2.) RT, 24 h; | 86% |
-
-
81693-14-3
5-(3E,6-heptadienyl)-dihydro-2(3H)-furanone

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In hexane at 16℃; for 1h; |

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In benzene for 5h; Heating; | 58% |
| With dibenzoyl peroxide In benzene Heating; | 58% |

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| Stage #1: 1-cyclohexyloxy-undec-1-yn-4-ol With hydrogenchloride In tetrahydrofuran at 20℃; Hydrolysis; Stage #2: With sodium hydride In tetrahydrofuran at 0 - 20℃; Cyclization; Further stages.; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 66 percent 2: 64 percent / Bu3N, HCOOH / Pd(OAc)2*3(PPh2) / dimethylformamide / 7 h / 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether / 1 h / -30 - 20 °C 2: C21H12F9P*C8H18NO4S2(1-)*Au(1+); 3-chloro-benzenecarboperoxoic acid; methanesulfonic acid / 1,2-dichloro-ethane / 5 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: diethyl ether / 1 h / -30 - 20 °C 2.1: n-butyllithium / diethyl ether; hexane / 0.75 h / 0 - 20 °C 2.2: 0 - 20 °C 3.1: C21H12F9P*C8H18NO4S2(1-)*Au(1+); methanesulfonic acid / chloroform-d1 / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; tetraethylammonium tosylate In N,N-dimethyl-formamide Ambient temperature; electroreductive crossed hydrocoupling; | 77% |

| Conditions | Yield |
|---|---|
| nickel(II) iodide; samarium diiodide In tetrahydrofuran for 1h; Addition; | 83% |

-
A
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran at 20℃; for 1h; Hydrolysis; |
-
-
1396569-87-1
C11H19(2)HO

-
-
937-14-4
3-chloro-benzenecarboperoxoic acid

-
A
-
1396571-80-4
C18H24(2)HClO4

-
B
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; C21H12F9P*C8H18NO4S2(1-)*Au(1+) In chloroform-d1 at 20℃; |

-
-
116625-66-2
5-heptyl-tetrahydro-furan-2-ol

-
-
937-14-4
3-chloro-benzenecarboperoxoic acid

-
A
-
1396571-72-4
C18H25ClO4

-
B
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In 1,2-dichloro-ethane at 20℃; for 5h; | A 90 %Spectr. B 10 %Spectr. |

-
-
116625-68-4
2-heptyl-2,3-dihydrofuran

-
-
937-14-4
3-chloro-benzenecarboperoxoic acid

-
A
-
1396571-72-4
C18H25ClO4

-
B
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; water In 1,2-dichloro-ethane at 20℃; for 5h; Reagent/catalyst; | A 21 %Spectr. B 33 %Spectr. |


-
A
-
68820-32-6
(Z)-4-undecenal

-
B
-
68820-35-9
(E)-undec-4-enal

-
C
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With ethyl acetate In tetrahydrofuran; ethanol at 490℃; for 2.5h; Inert atmosphere; optical yield given as %de; | A n/a B n/a C 30% |


-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride | 84% |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 101 g / diethyl ether; tetrahydrofuran / 4 h / -5 °C 2: Na / diethyl ether / 1.5 h / Heating 3: pyridinium chlorochromate / CH2Cl2 / 1.25 h / Heating 4: 3-(2-ethoxyethyl)-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide / triethylamine; dioxane / 15 h / 87 °C 5: 1.) NaOH, 2.) NaBH4 / 1.) EtOH-H2O, reflux, 0.5 h, 2.) 2 h 6: 0.5 g / 110 deg C; -> 145 deg C, 20 min; 145 deg C, 10 min 7: H2 / 10percent Pd/C / hexane / 1 h / 16 °C View Scheme |
-
-
81651-44-7
2-Allyl-3-chlor-2H-3,4,5,6-tetrahydropyran

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: Na / diethyl ether / 1.5 h / Heating 2: pyridinium chlorochromate / CH2Cl2 / 1.25 h / Heating 3: 3-(2-ethoxyethyl)-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide / triethylamine; dioxane / 15 h / 87 °C 4: 1.) NaOH, 2.) NaBH4 / 1.) EtOH-H2O, reflux, 0.5 h, 2.) 2 h 5: 0.5 g / 110 deg C; -> 145 deg C, 20 min; 145 deg C, 10 min 6: H2 / 10percent Pd/C / hexane / 1 h / 16 °C View Scheme |
-
-
81651-45-8
octa-4trans,7-dien-1-ol

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: pyridinium chlorochromate / CH2Cl2 / 1.25 h / Heating 2: 3-(2-ethoxyethyl)-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide / triethylamine; dioxane / 15 h / 87 °C 3: 1.) NaOH, 2.) NaBH4 / 1.) EtOH-H2O, reflux, 0.5 h, 2.) 2 h 4: 0.5 g / 110 deg C; -> 145 deg C, 20 min; 145 deg C, 10 min 5: H2 / 10percent Pd/C / hexane / 1 h / 16 °C View Scheme |
-
-
56053-82-8
octadiene-4(E),7 al-1

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 3-(2-ethoxyethyl)-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide / triethylamine; dioxane / 15 h / 87 °C 2: 1.) NaOH, 2.) NaBH4 / 1.) EtOH-H2O, reflux, 0.5 h, 2.) 2 h 3: 0.5 g / 110 deg C; -> 145 deg C, 20 min; 145 deg C, 10 min 4: H2 / 10percent Pd/C / hexane / 1 h / 16 °C View Scheme |
-
-
90162-80-4
(E)-hydroxy-7,10-undecadienoic acid

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 0.5 g / 110 deg C; -> 145 deg C, 20 min; 145 deg C, 10 min 2: H2 / 10percent Pd/C / hexane / 1 h / 16 °C View Scheme |
-
-
90162-79-1
ethyl (E)-4-oxoundeca-7,10-dienoate

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) NaOH, 2.) NaBH4 / 1.) EtOH-H2O, reflux, 0.5 h, 2.) 2 h 2: 0.5 g / 110 deg C; -> 145 deg C, 20 min; 145 deg C, 10 min 3: H2 / 10percent Pd/C / hexane / 1 h / 16 °C View Scheme |
-
-
124-13-0
Octanal

-
-
37651-49-3
diethyl-(2-methoxycarbonyl-ethyl)-methyl-ammonium; iodide

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With tetraethylammonium tosylate In N,N-dimethyl-formamide cathodic reduction; | 46% |
-
-
35329-48-7
trans-Nonenylmalonsaeure

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With sulfuric acid In water for 10h; Heating; |
-
-
114702-12-4
4-Hydroxy-undec-2-ynoic acid ethyl ester

-
A
-
100591-76-2
5-heptyl-dihydro-furan-2-one

-
B
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With formic acid; bis(triphenylphosphine) palladium (Il) acetate; tributyl-amine In N,N-dimethyl-formamide at 60℃; Product distribution; variation excess of formic acid; | |
| With formic acid; tributyl-amine; Pd(OAc)2*(PPh2) In N,N-dimethyl-formamide at 60℃; for 7h; Product distribution; ammount of HCOOH; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 28 percent / LDA / tetrahydrofuran / -78 - 20 °C 2: 87 percent / CF3CO3H / CH2Cl2 / 6 h / 0 °C View Scheme |

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 73 percent / ozone, methanolic NaOH / CH2Cl2 / -70 °C 2: 86 percent / NaBH4, Na2HPO4*12H2O / methanol / 1.) 0 deg C, 5 min, 2.) RT, 24 h View Scheme |
-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

-
-
116625-66-2
5-heptyl-tetrahydro-furan-2-ol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In toluene at -78℃; for 2h; Reduction; | 100% |
| With diisobutylaluminium hydride | |
| With diisobutylaluminium hydride In toluene | |
| Stage #1: 5-heptyldihydro-2(3H)-furanone With diisobutylaluminium hydride In tetrahydrofuran at -78℃; Stage #2: In water Acidic aq. solution; | |
| Multi-step reaction with 2 steps 1: [(1,3-bis(2,4,6-trimethylphenyl) imidazol-2-ylidene)Fe(CO)4] / toluene / 3 h / 20 °C / Schlenk technique; Inert atmosphere; UV-irradiation 2: hydrogenchloride; water / toluene; tetrahydrofuran / 2 h / 20 °C / Schlenk technique; Inert atmosphere View Scheme |
-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In benzene at 20℃; for 10h; Chlorination; | 100% |

| Conditions | Yield |
|---|---|
| With hafnium tetrakis(trifluoromethanesulfonate); palladium on activated carbon; hydrogen In neat (no solvent) at 135℃; under 760.051 Torr; for 12h; Schlenk technique; Glovebox; | 99% |
| With palladium 10% on activated carbon; W(OTf)6; hydrogen at 135℃; under 760.051 Torr; for 12h; | 90% |
| With palladium on activated carbon; W(OTf)6; hydrogen In neat (no solvent) at 135℃; under 760.051 Torr; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride for 37h; Ambient temperature; | 91.3% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol; tert-butyl alcohol Heating; | 91% |
| With diphenylsilane; triphenylphosphine; chloro(1,5-cyclooctadiene)rhodium(I) dimer In tetrahydrofuran at 20℃; | 63% |
| With copper chromite; hydrogen at 210 - 220℃; under 125 - 150 Torr; | |
| With lithium aluminium tetrahydride | |
| With diphenylsilane; triphenylphosphine; chloro(1,5-cyclooctadiene)rhodium(I) dimer In tetrahydrofuran at 20℃; for 72h; Reduction; | 63 % Spectr. |

| Conditions | Yield |
|---|---|
| Stage #1: 5-heptyldihydro-2(3H)-furanone; formic acid ethyl ester With potassium methanolate In diethyl ether at 15 - 20℃; for 3h; Inert atmosphere; Stage #2: With acetic acid In diethyl ether; water at 20℃; for 0.5h; Inert atmosphere; | 61% |
| With sodium hydride In diethyl ether at 20℃; for 3h; |
-
-
58133-26-9
1,1-dibromohexane

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

-
A
-
109586-27-8
10-Hydroxy-heptadecan-7-one

-
B
-
109586-25-6, 109586-26-7
5-heptyl-2-hexylidene-2,3,4,5-tetrahydrofuran

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; titanium tetrachloride; zinc In tetrahydrofuran at 25℃; for 1.5h; | A 21% B 51% |

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

-
-
22847-06-9
4-oxoundecanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-heptyldihydro-2(3H)-furanone With water; sodium hydroxide In ethanol at 20℃; for 12h; Inert atmosphere; Stage #2: With Dess-Martin periodane In ethanol; dichloromethane at 20℃; for 5h; Inert atmosphere; | 46% |
| Multi-step reaction with 2 steps 1: NaOH / ethanol 2: NaOCl / aq. phosphate buffer / 20 h / 20 °C / pH 6.6 View Scheme |
-
-
557-91-5
1,1-Dibromoethane

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

-
A
-
109586-24-5
6-Hydroxy-tridecan-3-one

-
B
-
120387-80-6
2-ethylidene-5-heptyl-2,3,4,5-tetrahydrofuran

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; titanium tetrachloride; zinc In tetrahydrofuran at 25℃; for 2h; | A 41% B 43% |

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

-
-
116625-68-4
2-heptyl-2,3-dihydrofuran

| Conditions | Yield |
|---|---|
| Stage #1: 5-heptyldihydro-2(3H)-furanone With diisobutylaluminium hydride In dichloromethane at -78℃; for 2h; Inert atmosphere; Stage #2: With methanesulfonyl chloride; triethylamine In tetrahydrofuran at -50℃; for 8h; Reflux; | 42% |
| Multi-step reaction with 3 steps 1: DIBAH 2: BF3*OEt2 3: 58 percent / tBuOOH, Ti(iPrO)4, iPr2NEt / CH2Cl2 / 25 h / 0 °C View Scheme |
-
-
31729-70-1
bis(iodozinc)methane

-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; titanium chloride In tetrahydrofuran at 25℃; for 4h; methylenation; | 28% |
-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With methanol; ammonia |
-
-
104-67-6
5-heptyldihydro-2(3H)-furanone

| Conditions | Yield |
|---|---|
| With copper chromite at 280℃; Hydrogenation.unter Druck und folgenden Verestern mit Capronsaeure in Gegenwart von Naphthalinsulfonsaeure bei 205grad; |
Undecan-4-olide Consensus Reports
Undecan-4-olide Specification
The CAS registry number of Undecan-4-olide is 104-67-6. Its EINECS registry number is 203-225-4. The IUPAC name is 5-heptyloxolan-2-one. In addition, the molecular formula is C11H20O2 and the molecular weight is 184.28. It is also called dihydro-5-heptyl-2(3H)-furanone. What's more, it is a kind of clear colourless liquid. It should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 2.92; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.92; (4)ACD/LogD (pH 7.4): 2.92; (5)ACD/BCF (pH 5.5): 96.85; (6)ACD/BCF (pH 7.4): 96.85; (7)ACD/KOC (pH 5.5): 918.85; (8)ACD/KOC (pH 7.4): 918.85; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 6; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.449; (14)Molar Refractivity: 52.67 cm3; (15)Molar Volume: 196.3 cm3; (16)Polarizability: 20.88 ×10-24cm3; (17)Surface Tension: 31.3 dyne/cm; (18)Density: 0.938 g/cm3; (19)Flash Point: 112.7 °C; (20)Enthalpy of Vaporization: 52.51 kJ/mol; (21)Boiling Point: 286 °C at 760 mmHg; (22)Vapour Pressure: 0.00271 mmHg at 25°C.
Preparation of Undecan-4-olide: it can be prepared by undecenoic acid in the presence of sulfuric acid through esterification reaction. And then after a series of separation, washing and distillation you can get the desired product. In addition, it can be prepared by cyclopropylideneoctane. This reaction will need reagent CF3CO3H and solvent CH2Cl2. The reaction time is 6 hours at reaction temperature of 0 °C. The yield is about 87%.
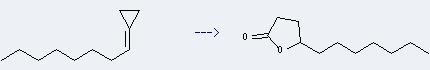
Uses of Undecan-4-olide: it is used as a food flavor. And it can be used to get 5-heptyl-tetrahydro-furan-2-ol. This reaction will need reagent i-Bu2AlH and solvent toluene. The reaction time is 2 hours at reaction temperature of -78 °C. The yield is about 100%.
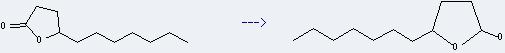
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1OC(CCCCCCC)CC1
(2)InChI: InChI=1/C11H20O2/c1-2-3-4-5-6-7-10-8-9-11(12)13-10/h10H,2-9H2,1H3
(3)InChIKey: PHXATPHONSXBIL-UHFFFAOYAL
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 18500mg/kg (18500mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
Related Products
- Undecan-4-olide
- 104678-13-9
- 104678-68-4
- 1046788-84-4
- 104680-36-6
- 1046816-12-9
- 10468-17-4
- 1046832-15-8
- 1046862-09-2
- 10469-09-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View